Risk of Systemic Disorders in Patients with Periodontal Infection
Shima Afrasiabi*
Department of Microbiology, School of Medicine, Tehran University of Medical Sciences, Iran
*Corresponding author: Shima Afrasiabi, Medical Bacteriology, Department of Microbiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Article History
Received: March 15, 2021 Accepted: April 14, 2021 Published: April 16, 2021
Citation: Afrasiabi S. Risk of Systemic Disorders in Patients with Periodontal Infection. Int. J. Orl. Health. 2021;1(1):20‒22. DOI: 10.51626/ijoh.2021.01.00005
Abstract
A number of diverse studies have indicated that there is a link between periodontal infection and systemic diseases, such as cardiovascular diseases, diabetes mellitus, adverse pregnancy outcomes, mouth cancer, rheumatoid arthritis and respiratory infections. This study discusses the correlation between periodontal infections and systemic disorders. Periodontal infections should be considered as an important risk factor for various systemic diseases.
Keywords: Periodontal diseases, Cardiovascular diseases, Diabetes mellitus, Respiration disorders, Rheumatoid Arthritis
Introduction
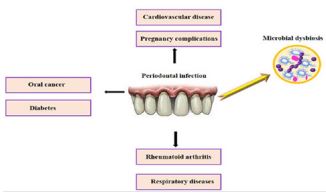
Periodontal disease is a multifactorial condition with inflammatory challenge of the periodontium at a systemic level, so that the epithelium surface that could be ulcerated in the periodontal pockets [1]. The most common bacterial causes of periodontal disease include Porphyromonas gingivalis, Actinobacillus actinomyceyemcomitans, Tannerella forsythensis and Treponema denticola [2]. Periodontal disease causes inflammation of the gums, leading to the gradual destruction of periodontal tissues and alveolar bone supporting the teeth [3]. Periodontal pathogens, are known to produce endotoxins could enhance development of non-oral systemic disease, including cardiovascular diseases, mouth cancer, diabetes, rheumatoid arthritis, respiratory diseases, and complications of pregnancy (Figure 1) [4]. This study attempts to summarize the available evidence on the role of periodontal infection in the development of systemic disorders.
Figure 1: Relationship between periodontal infection and systemic disorders.
Relationship between periodontal infection and cardiovascular disease
In patients with periodontal infection, the bacteremia was detected in a number of patients after dental extraction, periodontal surgery, tooth scaling and tooth brushing [5]. Due to periodontal infection, inflammatory mediators can enter the bloodstream, which is associated with an increased risk of coronary artery disease (CAD) [6]. Periodontal infection is associated with elevated platelets, which can lead to an increased risk of thrombosis [7, 8]. Porphyromonas gingivalis is an anaerobic bacterium that contributes to inflammation and tissue loss during periodontal disease. It then stops apoptosis via the PI3K/Akt and JAK/Stat signaling pathways, allowing the proliferation of intracellular pathogens. P. gingivalis can affect complement and antimicrobial agents and it is likely to contribute to the development of atherosclerosis [9]. P. gingivalis can cause impaired in gut microbiota, which leads to systemic inflammation [10].
Relationship between periodontal infection and diabetes
Diabetes mellitus is a group of metabolic disease manifested by hyperglycemia in the blood caused by impaired in insulin secretion and/or insulin action [11]. Lipopolysaccharide (LPS) of bacteria that causes periodontitis through Toll-Like Receptors (TLRs) cause pro-inflammatory factors interleukin 1β (IL-1ß) and Tumor necrosis factor alpha (TNFα) from the host as well as the serum levels of interleukin 6 (IL-6) and C-reactive protein (CRP) have also increased in patients with periodontal disease, so that periodontal disease can lead to increased insulin resistance. There is an increase of IL-6 and CRP production in patients with periodontal disease compared to healthy people [12,13]. Increased inflammatory agents, result in higher levels of insulin resistance [14].
Relationship between periodontal infection and pregnancy complications
Physiological changes in pregnancy change microbial communities of mucosal surfaces which induce pro-inflammatory markers [15]. It has been observed that oral infection could increase the risk of preterm or low birth weight [16]. Periodontal infection is associated with elevated circulatory endotoxin. Enteric endotoxins can induce spontaneous abortions, fetal organ damage, placental necrosis, fetal death and malformations [17]. LPS can stimulate the production of cytokines IL-1ß, TNF-α, IL-6, prostaglandin E2 (PGE2) and matrix metalloproteinases [18]. The local production of these cytokines in the periodontal pocket caused by periodontal infection may also result in an elevated serum concentration of such cytokines [19]. These components can enter the bloodstream and cross the placental barrier and therefore also be present in the amniotic fluid. If the levels of PGE2 and TNF-α increase in the amniotic fluid, can induce a preterm birth [20]. Infections during pregnancy can lead to serious consequences, such as preterm birth, fetal death and injury [21].
Relationship between periodontal infection and mouth cancer
Mouth cancer, characterized by gingival squamous cell carcinoma in particular, and showing symptoms of bleeding, swelling, deep periodontal pocket, and bone destruction [22]. Bacteria may play a more direct role in carcinogenesis in the mouth [23]. Attacking the periodontal supporting tissues occurs during periodontitis, resulting in constant low grade systemic inflammation indicated by the increased expression of circulating inflammatory responses [24]. Periodontal infection has a direct or indirect effect in on the carcinogenic process. Direct involvement occurs through endotoxins and enzymes of microorganisms. They can directly induce mutations in tumor suppressor genes or alter signaling pathway that affect cell proliferation and survival of epithelial cells. The indirect effect of periodontal disease on mouth cancer described through inflammatory cells [25].
Relationship between periodontal infection and rheumatoid arthritis
There is evidence that periodontal disease and rheumatoid arthritis, a chronic inflammatory disease, share major known risk factors such as the HLA-DRB1 allele. This association may also be through the activity of major periodontal pathogens. Specifically, P. gingivalis can induce protein citrullination by releasing a deaminase, which through a mimicry process might stimulate anti-citrullinated protein antibodies formation in rheumatoid arthritis patients [26,27].
Relationship between periodontal infection and respiratory diseases
There is further evidence that A. actinomycetemomitans and P. gingivalis may cause aspiration pneumonia when aspirated into the lung. Periodontal disease-associated enzymes in saliva may promote adherence of respiratory pathogens to respiratory tract mucosal surfaces, thereby causing infection. Oral bacteria contain enzymes or cytokines that alter the respiratory epithelium and enhance infection by respiratory pathogens [28].
Conclusion
Although many details of the mechanisms underlying the association between periodontal infection and systemic disorders are still unclear, available review has highlighted the important links between the two chronic situations. It is already clear that management of periodontal disease and good oral hygiene can promote the general health.
References
- Agueda A, Echeverría Manau A, Manau C (2008) Association between periodontitis in pregnancy and preterm or low birth weight: Review of the literature. Med Oral Patol Oral Cir Bucal 13(9): E609-615.
- Pihlstrom BL, Michalowicz BS, Johnson NW (2005) Periodontal diseases. The lancet 366(9499): 1809-1820 .
- Irfan U, Dawson D, Bissada N (2001) Epidemiology of periodontal disease: a review and clinical perspectives. J Int Acad Periodontol 3(1): 14-21.
- Ohyama H, Nakasho K, Yamanegi K, Noiri Y, Kuhara A, et al . (2009) An unusual autopsy case of pyogenic liver abscess caused by periodontal bacteria. Jpn J Infect Dis 62(5): 381-383.
- Maharaj B, Coovadia Y, Vayej AC (2012) An investigation of the frequency of bacteraemia following dental extraction, tooth brushing and chewing. Cardiovasc J Afr 23(6): 340.
- Holmlund A, Lampa E, Lind L (2017) Oral health and cardiovascular disease risk in a cohort of periodontitis patients. Atherosclerosis 262: 101-106.
- Zhan Y, Lu R, Meng H, Wang Xe, Hou J (2016) Platelet activation and platelet–leukocyte interaction in generalized aggressive periodontitis. J Leukoc Biol 100(5): 1155-1166.
- Papapanagiotou D, Nicu EA, Bizzarro S, Gerdes VE, Meijers JC, Nieuwland R, et al. (2009) Periodontitis is associated with platelet activation. Atherosclerosis 202(2): 605-611.
- Hussain M, Stover CM, Dupont A (2015) P. gingivalis in periodontal disease and atherosclerosis–scenes of action for antimicrobial peptides and complement. Front Immunol 6: 45.
- Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, et al. (2014) Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep 4(1):1-9.
- Zhou X, Zhang W, Liu X, Zhang W , Li Y (2015) Interrelationship between diabetes and periodontitis: role of hyperlipidemia. Arch Oral Biol 60(4): 667-674.
- Mesia R, Gholami F, Huang H, Clare-Salzler M, Aukhil I, Wallet SM, et al. (2016) Systemic inflammatory responses in patients with type 2 diabetes with chronic periodontitis. BMJ Open Diabetes Res Care 4(1): e000260.
- Preshaw P, Alba A, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. (2012) Periodontitis and diabetes: a two-way relationship. Diabetologia 55(1): 21-31.
- Teeuw WJ, Gerdes VE, Loos BG (2010) Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes care 33(2): 421-427.
- Lunardelli AN, Peres MA (2005) Is there an association between periodontal disease, prematurity and low birth weight? A population‐based study. J Clin Periodontol 32(9): 938-946.
- Manau C, Echeverria A, Agueda A, Guerrero A, Echeverria JJ (2008) Periodontal disease definition may determine the association between periodontitis and pregnancy outcomes. J Clin Periodontol 35(5): 385-397.
- Haesaert B, Ornoy A (1986) Transplacental effects of endotoxemia on fetal mouse brain, bone, and placental tissue. Pediatr Pathol 5(2): 167-181.
- Offenbacher S, Beck J, Lieff S, Slade G (1998) Role of periodontitis in systemic health: spontaneous preterm birth. J Dent Educ 62(10): 852-858.
- Collins J, Windley Hr, Arnold R, Offenbacher S (1994) Effects of a Porphyromonas gingivalis infection on inflammatory mediator response and pregnancy outcome in hamsters. Infect Immun 62(10): 4356-4361.
- Li X, Kolltveit KM, Tronstad L, Olsen I (2000) Systemic diseases caused by oral infection. Clin Microbiol Rev 13(4): 547-558.
- Zi MYH, Longo PL, Bueno-Silva B, Mayer MPA (2015) Mechanisms involved in the association between periodontitis and complications in pregnancy. Front Public Health 2: 290.
- Seoane J, Varela‐Centelles PI, Walsh TF, Lopez‐Cedrun JL, Vazquez I, et al. (2006) Gingival squamous cell carcinoma: diagnostic delay or rapid invasion? J Periodontol 77(7): 1229-1233.
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917): 860-867.
- Moutsopoulos NM, Madianos PN (2006) Low‐grade inflammation in chronic infectious diseases: Paradigm of periodontal infections. Ann N Y Acad Sci 1088(1): 251-264.
- Tezal M, Sullivan MA, Reid ME, Marshall JR, Hyland A, Loree T, et al. (2007) Chronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg 133(5): 450-454.
- Aviña‐Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D (2008) Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Arthritis Res Ther 59(12): 1690-1697.
- Rodríguez-Lozano B, González-Febles J, Garnier-Rodríguez JL, Dadlani S, Bustabad-Reyes S, Sanz M, et al. (2019) Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: a case–control study. Arthritis Res Ther 21(1): 1-12.
- Gomes-Filho IS, Passos JS, Seixas da Cruz S (2010) Respiratory disease and the role of oral bacteria. J Oral Microbiol 2(1): 5811.