Screening for Small for Gestational Age Fetus: Are we Succeeding? an Audit of Performance at a Regional Hospital
Nahian A, Mahomed K* and Fraser C
Department of Obstetrics, Ipswich Hospital, Australia
*Corresponding author: Kassam Mahomed, Senior Staff Specialist, Department of Obstetrics and Gynecology, Ipswich Hospital and University of Queensland, Chelmsford Avenue, Ipswich, QLD 4305, Australia.
Article History
Received: April 12, 2021 Accepted: April 22, 2021 Published: April 23, 2021
Citation: Nahian A, Mahomed K, Fraser C. Screening for Small for Gestational Age Fetus: Are we Succeeding? an Audit of Performance at a Regional Hospital. Int. J.Obst & Gync. 2021;1(1):01‒06. DOI: 10.51626/ijog.2021.01.00001
Abstract
Background: Fetal growth restriction affects perinatal morbidity and mortality and in practice. symphysis fundal height measurement only identifies 17.3-24.8% of Small for Gestational Age (SGA) fetuses. Plotting on a growth chart and observation of trends has been reported to increase detection rate. Failure to diagnose such fetuses is a recurrent issue at the hospital.
Aims: To determine rate of detection of SGA fetuses during the antenatal period and to determine effect of maternal factors on the detection rate.
Materials and Methods: This was a retrospective chart review of all babies born at Ipswich General Hospital from 2016 to 2018 in singleton pregnancies with birthweight below the tenth percentile and born ≥28 weeks gestation confirmed by first trimester dating ultrasound.
Results: Over 3 years, 760 singleton SGA infants were born. 86.4% had at least 3 antenatal visits after 20 weeks gestation. Although at some stage in pregnancy 52.5% had a symphysis fundal height (SFH) measurement ≥3cm less than gestational age, in only 273 (36%) this resulted in a request for an ultrasound scan to confirm SGA fetus. This happened 53.1% of women who had a scan. Statistical analysis showed the only factor to affect SFH measurement and ultrasound diagnosis of SGA was maternal BMI while maternal age, parity and smoking status had no significant effect.
Conclusion: The rate of detection of SGA is low but may be improved with the introduction of a new program of education and plotting of SFH on charts to assess trend.
Keywords: Small for gestational age; Symphysis-Fundal height; Antenatal care; Ultrasound
Impact Statement
What is already known on this subject?
FGR is a serious condition that affects a significant number of pregnancies. It is a major cause of stillbirth even in high income countries and the risk of perinatal mortality is greater in undetected cases of SGA, even in low-risk populations. FGR is often difficult to diagnose in the antenatal setting, both due to the lack of sensitivity of the clinical tools such as SFH used to detect SGA as well as due to clinician and patient factors.
What do the results of this study add?
Our study shows that despite attendance to an antenatal care at a specialist facility, the diagnosis of SGA is often missed. We have identified both patient factors such as maternal BMI and clinician factors such as inconsistency in measurement of SFH that contribute to this.
What are the implications of these findings for clinical practice and/or further research?
The identification of patient factors that affect the detection of SGA are often static variables that cannot or should not be corrected during pregnancy however they can aid in risk stratification. The implication of the findings of these clinician factors contributing to SGA detection is to guide the introduction of a new program of education and clinical practice which may result in an increased detection of SGA pregnancies.
Introduction
Fetal Growth Restriction (FGR) affects 5-10% of pregnancies [1]. The detection of FGR is one of the major components of antenatal care as these fetuses are at increased risk of perinatal mortality and morbidity related to preterm birth, perinatal asphyxia and hypoglycaemia and requiring admission to special care nursery [2]. Early detection of FGR allows the opportunity to reduce morbidity and mortality through close monitoring and timely delivery [3]. In practice, screening for FGR requires identification of a fetus that is Small for Gestational Age (SGA), defined as Estimated Fetal Weight (EFW) below the 10th percentile [3] and then differentiation between fetuses that are constitutionally small but healthy with appropriate fetal size for maternal size and ethnicity or growth restricted as defined according to the Delphi consortium [4].
As abdominal palpation has limited accuracy in detection of SGA fetuses, detecting only 21% of SGA fetuses [5], Symphysis-Fundal Height (SFH) measurement is recommended at each antenatal visit. Single SFH measurements however only identify 17.3-24.8% of SGA babies [6,7] but there is advocation for serial SFH measurement and plotting on a growth chart and observation of trends has been reported to increase the rate of detection to approximately 50% [8]. Selective ultrasound scan based on SFH measurement, as opposed to routine scanning of all mothers in the third trimester, can then be used to confirm diagnosis [9]. Failure to diagnose SGA fetuses is a recurrent issue in perinatal morbidity and mortality meetings at Ipswich Hospital. The primary objective of this study was to ascertain what percentage of the babies born with a birthweight below the tenth centile of gestational age had been suspected and/or identified during the antenatal period. The secondary objective was to determine the effect of patient factors on rate of detection of SGA.
Materials and Methods
This was a retrospective review of all babies born at Ipswich Hospital, Ipswich, Queensland, Australia. This is a level 2 hospital with a capability framework to look after women who birth at 32 weeks and above. We included all singleton pregnancies with babies born with a birthweight below the 10th centile for Gestational Age (GA) and born at ≥28 weeks gestation confirmed by first trimester dating ultrasound. These were manually extracted from the Birth Suite Register. We excluded pregnancies where there was lethal congenital malformation. All medical records were reviewed by one member of the research team. Data collected included maternal and pregnancy characteristics, information on antenatal care and on labour and birth outcomes.
Statistical Analysis
The Differences between the two groups in relation to maternal characteristics were tested using Student’s t test for normally distributed continuous data. Differences between proportions for categorical data was tested using Chi-square test, otherwise Fisher’s exact test was used when there was a count of 5 or less. The relationship between test status and each maternal characteristic and pregnancy outcome variable was initially explored using univariate logistic regression. Maternal characteristics found to have a statistically significant relationship with SGA status were incorporated into the multivariate logistic regression models examining the association between SGA status and each of the variables. Statistical significance was set at a two-sided p value of <0.05. Stata/SE version 9.0 for Windows (Stata Corp). The study was considered by the Chair of the West Moreton Research Ethics Committee who deemed the study to be a quality activity involving an audit of practice and therefore exempt from full ethical review Ex46-20.
Results
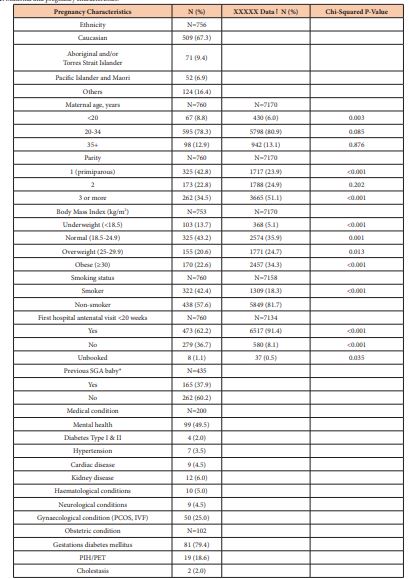
A total of 760 singleton infants with SGA were born to 729 women during the three-year period. Twenty-nine women had more than one pregnancy during the study period; 27 women had two pregnancies and 2 women had three pregnancies. With regard to our primary objective, overall, in the 760 women birthing with an SGA baby during the period a total of 186 (23.8%) were confirmed antenatally. If we look at the 650 pregnancies with at least 3 antenatal visits after 20 weeks gestation, confirmation of SGA was made in 28.6% of pregnancies. In a total of 405 ultrasounds performed, 186 (45.9%) confirmed the diagnosis of SGA. The maternal demographic characteristics are shown in Table 1. We compared some of these variables against women with singleton pregnancies who did not have an SGA baby who birthed at ≥28 weeks gestation during the same period 2016-2018. Caucasian women comprised 509 (67.3%) of the study population and Aboriginal and Torres Strait Islander women comprised 71 (9.4%). The other variables shown are for women during each of the pregnancy and include the 29 multiparous women. Maternal age grouping was similar to the hospital population however there were more mothers <20 years in the SGA population. There were 325 (42.8%) births in women in their first pregnancy and 262 (34.5%) in women with 3 or more pregnancies. In contrast there were fewer first and more 3rd or more pregnancies in the hospital population. In terms of BMI, 325 (43.2%) women were overweight or obese, fewer than 59% for the hospital population. 322 (42.4%) women reported smoking in pregnancy, significantly higher than in the hospital population where 1309 (18.3%) reported smoking in pregnancy. Of the 435 women in their second or subsequent pregnancy 165 (37.9%) had had a previous SGA baby. First hospital antenatal visit was after 20 weeks gestation in 279 (36.7%) women. Mental ill health was documented in 99 (13%) of the women although not all were medicated.
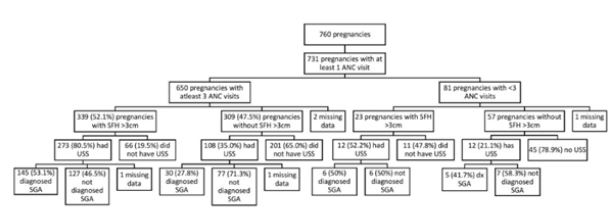
The mean number of antenatal visits was 5.4 and as seen in Figure 1, 731 pregnancies were associated with at least one hospital antenatal visit. 650 pregnancies (86.4%) were associated with at least 3 hospital antenatal visits after 20 weeks gestation. Of the 339 (52.5%) with SFH measurement 3cms or more less than expected for gestational age, in 66 (19.5%), this was not followed up by a growth scan despite the fact that a record had been made of an abnormal SFH. In the remaining 273 (80.5%), the scan confirmed SGA in 145 (53.1%) cases. In 309 (47.5 %), SFH was not noted to be less than 3cm but a scan for growth was requested (possibly to screen for fetal growth restriction for a clinical indication) in 108 (35.0%) of these cases with confirmation of SGA in 30 (27.8%) of these 108 pregnancies. In the group of 650 pregnancies with at least 3 antenatal visits after 20 weeks gestation, an ultrasound confirmation of SGA was made in 186 (28.6%) pregnancies. In a total of 405 ultrasounds performed, 186 (45.9%) confirmed the diagnosis of SGA.
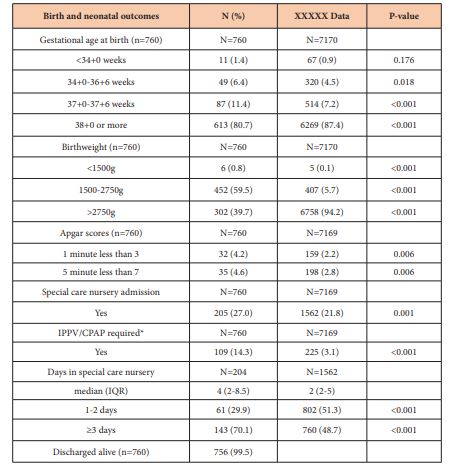
Planned delivery by induction of labour or caesarean section was performed in 141 (18.6%) of the 760 pregnancies and in 130 (69.9%) of pregnancies where an ultrasound diagnosis of SGA was made. Out of all 760 pregnancies, placental abruption occurred in 7 (0.9%) cases, of whom 3 cases had had an ultrasound confirmation of SGA. Birth and neonatal outcomes are presented in Table 2 and we have again compared these to the defined hospital population. A total of 205 (27%) of neonates were admitted to special care nursery and 143 (70.1%) of them spent 3 days or more. All the indicators of perinatal morbidity were significantly more common in the SGA babies compared to the normal hospital population. Of the 760 babies born, 4 (0.5%) were not discharged alive. 2 of these were born <3rd centile for gestational age. 1 (GA 35+2, birthweight 1750g) had antenatal suspicion of SGA with confirmation of diagnosis with ultrasound and was stillborn from placental abruption. The other (GA 35+0, birthweight 1600g) had only one antenatal care visit and no documented clinical suspicion of SGA despite a SFH measurement with >3cm difference and death was unexplained. Two babies were born 3rd-5th centile, both with no documented clinical suspicion of SGA. One of these (GA 38+3, birthweight 2480g) had no concerning SFH measurements over 2 ANC encounters and was born before arrival to hospital and delivered stillborn. The other (GA 38+4, birthweight 2476g) had SFH >3cm difference measured in one ANC encounter and had an unexplained death.
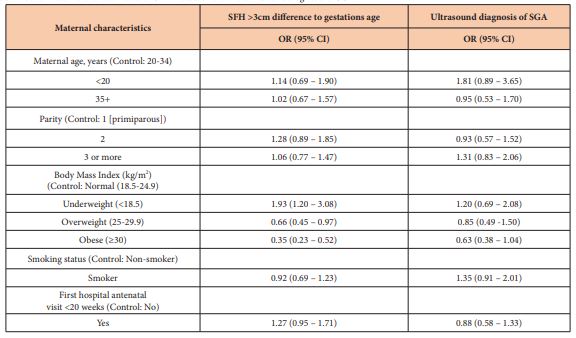
Our secondary objective was to determine the effect of maternal factors on detection of SGA fetus. As seen in Table 1, possible factors that need assessment are maternal age, parity, smoking status, BMI and gestational age at booking. As shown in table 3, there was no difference in SFH measurement of >3cm between mothers who were 20-34 versus ≥ 35 years old (OR 1.02, 95% CI 0.67 – 1.57) and no difference in rate of ultrasound diagnosis of SGA (OR 0.95, 95% CI 0.53 – 1.70). Between mothers who were smokers compared to nonsmokers, there was no difference in rate of SFH measurement >3cm to gestational age (OR 0.92, 95% CI 0.69 – 1.23) and in ultrasound diagnosis of SGA (OR 1.35, 95% CI 0.91 – 2.01). Maternal BMI had a significant effect on SFH measurement with the normal BMI group (BMI 18.5 – 24.9) being 1 to 2 times more likely to have a >3cm difference in SFH measurement than the overweight group (BMI 25 – 29.9) (OR 0.66, 95% CI 0.45 – 0.97) and 2 to 4 times more likely than the obese group (BMI ≥30) (OR 0.35, 95% CI 0.23 – 0.52). The underweight group (BMI <18.5) what 1 to 3 times more likely than the normal BMI group to have a >3cm difference in SFH measurement (OR 1.93, 95% CI 1.20 – 3.08).
Figure 1: Antenatal care and outcomes.
Table 1: Maternal and pregnancy characteristics.
PNO Data 2016-2018 (exclusion criteria: chromosomal abnormality, gestation
IPPV=Intermittent positive pressure ventilation CPAP=Continuous positive airway pressure.
Table 3: Effect of maternal characteristics on SFH measurement and ultrasound diagnosis of SGA.
Discussion
Detection of the SGA fetus during pregnancy is important because it is associated with poor perinatal outcome [11]. Firstly, we have documented that in only 273 (36%) out of the 760 pregnancies an abnormal SFH measurement suggestive of SGA was noted during the antenatal visit and resulted in a request for an ultrasound scan. Measurement of the SFH for fetal growth assessment is recommended over other methods such as the abdominal palpation that used to be standard practice in the past [3,12]. SFH was abnormal in 52.1% of cases but for some reason this did not lead to a request for a scan in 20% of these cases. We had no additional information to pursue this further. The fact that SFH was abnormal in only half of the pregnancies was not itself surprising as its sensitivity to detect SGA is low ranging from 0.27 to 0.76, which means it potentially fails to identify over 70% of pregnancies affected by SGA [13]. This high false negative rate has also been reported by others [6,7]. We were unable to determine why this would have been so, but we assume that just the measurement of SFH and recording onto the pregnancy record may not have alerted the clinician that there may have been something wrong. In addition, during the period when cases were assessed, measurement of SFH had not been consistent. Intra observer variation and inaccuracy of measurement has been identified by others [14] and may also be due to factors such as maternal obesity. One of the factors that affect SFH sensitivity is high BMI which affects the accuracy of fundal height measurements as shown in our results were mothers of normal BMI were 2 to 4 times more likely than mothers with high BMI to have SFH measurements >3cm different to gestational age. As 43.2% of the women in our review were overweight or obese, this may have reduced recognition of SGA using fundal height measurements. There is also an associated reduced sensitivity of ultrasound for diagnosis of SGA in women with high BMI as noted also by others [15]. Based on SFH measurement, even in women who had acceptable number of antenatal visits, whilst there should have been a suspicion of SGA based on SFH measurements, as stated above, a scan was not requested in 20% of such cases. Subsequent to this study we have observed possible reasons for the inaccuracy of the measurement. These may include lack of clinician’s experience as it has been previously reported that less experienced professionals had less confidence in using the tape measure. In addition, it has been shown that methods of measuring SFH vary a lot irrespective of level of experience in identifying upper and lower landmarks, in the choice of where the measurement starts and in the numbers being visible on the tape during the measurement [16]. This need and emphasis on accuracy and consistency in measurement has been one of the United Kingdom’s National Health Service’s initiatives for the early detection of SGA with focus on the training of clinicians on SFH measurement. The standardization of using a reversed paper tape, meticulous identification of the fundus by gentle palpation, starting to measure from the variable point (the fundus) down to the symphysis pubis and taking just one reading [12]. The use of SFH charts with 5th and 95th centiles with serial measurements plotted at every antenatal visit as part of the growth assessment protocol has been shown to increase the detection of SGA [17] as this allows clinicians to see the trend in growth.
Secondly, we have confirmed some of the risk factors for a SGA baby; women in their first pregnancy, women who smoked cigarettes and women who had their first antenatal visit after 20 weeks gestation. These are not new and even though these are modifiable, they continue to occur as has been reported in an extensive review on this topic [18]. Thirdly, we noted that when SGA was suspected clinically and women had an ultrasound scan, SGA was confirmed in only 53.1% of cases. Studies have shown that EFW using ultrasound scans can be inaccurate by greater than 10% in 34% of cases [19]. Sources of inaccuracy include operator experience and training, poor optimization of the ultrasound image and fetal positioning within the maternal pelvis [20]. Ultrasounds performed within one week of delivery have greater accuracy in determining EFW [21] with accuracy decreasing when performed earlier in the third trimester [22]. A weakness of our study was that we did not assess timing of the scans and whether or not there was a follow up scan and future research on the effect of timing of ultrasound in detection of SGA in such women is warranted. The ultrasound diagnosis of SGA did lead to greater rates of obstetric intervention with induction of labour and caesarean section and admission to SCN as expected. There were similar rates of placental abruption and need for IPPV/CPAP with and without ultrasound diagnosed SGA. As this was an observational study with no control groups and because there was only one neonatal death in the confirmed SGA group of pregnancies, it is difficult to comment on effect of SGA on neonatal mortality based on our data, but the literature suggests that SGA is a risk factor for neonatal mortality [2]. Early detection and management can allow the opportunity for risk management and prevention of neonatal mortality and morbidity through obstetric and pediatric intervention [23].
In conclusion, the rate of detection of SGA in our cohort is low and similar to previous reports and analysis based on patient demographics and in general supports what is in the wider literature. We have recently introduced routine use of SFH charts with measurements being recorded at each antenatal visit. All clinicians have participated in an online training on the detection of SGA with clear guidance on how to measure SFH and on when and what actions need to be taken. We would recommend another future audit to see if this has resulted in a higher detection rate of SGA fetuses and whether this has had an impact on perinatal mortality and morbidity.
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethical Approval
The study was considered by the Chair of the West Moreton Research Ethics Committee who deemed the study to be a quality activity involving an audit of practice and therefore exempt from full ethical review Ex46-20.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Bamfo JEAK, Odibo AO (2011) Diagnosis and management of fetal growth restriction. J Pregnancy 2011: 640715.
- Jing Liu, Xiao-Feng Wang, Yan Wang, Hua-Wei Wang, Ying Liu (2014) The incidence rate, high-risk factors, and short- and long-term adverse outcomes of fetal growth restriction: a report from Mainland China. Medicine (Baltimore) 93 (27): e210.
- Royal College of Obstetrics and Gynaecologists, (2013) The investigation and management of the small-for-gestational-age fetus (Green-top guideline No. 31). London: RCOG.
- Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, et al. (2016) Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 48(3): 333-339.
- Bais JMJ, Eskes M, Pel M, Bonsel GJ, Bleker OP (2004) Effectiveness of detection of intrauterine growth retardation by abdominal palpation as screening test in a low risk population: an observational study. Eur J Obstet Gynecol Reprod Biol 116(2): 164-169.
- Sparks TN, Cheng YW, McLaughlin B, Esakoff TF, Caughey AB (2011) Fundal height: a useful screening tool for fetal growth? J Matern Fetal Neo Med 24(5): 708-712.
- Roex A, Nikpoor P, van Eerd E, Hodyl N, Dekker G (2012) Serial plotting on customised fundal height charts results in doubling of the antenatal detection of small for gestational age fetuses in nulliparous women. Aust NZ J Obstet Gyn 52(1): 78-82.
- Gardosi J, Francis A (1999) Controlled trial of fundal height measurement plotted on customized antenatal growth charts. Br J Obstet Gynaecol 106(4): 309-344.
- Bricker L, Medley N, Pratt JJ (2015) Routine ultrasound in late pregnancy (after 24 weeks’ gestation). Cochrane Database Syst Rev 2015(6): CD001451.
- West Moreton Hospital and Health Services. Perinatal mortality and morbidity meetings Departmental annual reports 2016 – 2018.
- Kady M, Gardosi J (2004) Perinatal mortality and fetal growth restriction. Best Prac Res Clin Obstet Gynaecol 18(3): 397- 410.
- National Institute for Health and Clinical Excellence [NICE] 2019. Antenatal care for uncomplicated pregnancies. Clinical Guideline (CG62 2008).
- Pay ASD, Wiik J, Backe B, Jacobsson B, Strandell A (2015) Symphysis- fundus height measurement to predict small-for-gestational -age status at birth: a systematic review. BMC Pregnancy Childbirth 15: 22.
- Jelks A, Cifuentes R, Ross MG (2007) Clinicians bias in fundal height measurement. Obstet Gynecol 110(4): 892-899.
- Dude AM, Davis B, Delaney K, Yee LM (2019) Identifying fetal growth disorders using ultrasound in obese nulliparous women. J Matern Fetal Neonatal Med 34(1): 1768-1773.
- Griffiths A, Pinto A, Margarit I (2008) A survey of methods used to measure symphysis fundal height. J Obstet Gynaecol 28(7): 692-94.
- Gardosi J, Giddings S, Clifford S, Wood L, Francis A (2013) Association between regional stillbirth rates in England and regional uptake of accreditation training in customised fetal growth assessment. BMJ Open 3(12): e003942.
- McCowan L, Morgan RP (2009) Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol 23(6): 779-93.
- Hargreaves K, Cameron M, Edwards H, Deane K (2011) Is the use of symphysis-fundal height measurement and ultrasound examination effective in detecting small or large fetuses? J Obstet Gynaecol 31(5): 380-383.
- Milner J, Arezina J (2018) The accuracy of ultrasound estimation of fetal weight in comparison to birth weight: a systemic review. Ultrasound 26(1): 32-41.
- Faschingbauer F, Raabe E, Heimrich J, Faschingbauer C, Schmid M, et al. (2016) Accuracy of sonographic fetal weight estimation: influence of the scan-to-delivery interval in combination with the applied weight estimation formula. Arch Gynecol Obstet 294(1): 487-493.
- Ciobanu A, Khan N, Syngelaki A, Akolekar R, Nicolaides KH (2019) Routine ultrasound at 32 vs 36 weeks’ gestation: prediction of small-for-gestational-age neonates. Ultrasound Obstet Gynecol 53(6): 761-768.
- Lausman A, Kingdom J, Maternal Fetal Medicine Committee (2013) Intrauterine growth restriction: screening, diagnosis and management. J Obstet Gynecol Can 35(8): 741-748.