Acute Myocardial Infarction in a Healthy 57-Year-Old Female; A Case Report
Manisha Das*, Wilbert S Aronow
Department of Internal Medicine, University of Cincinnati, USA
*Corresponding author: Manisha Das, Division of Cardiovascular Disease, Department of Internal Medicine, USA. Dr Wilbert Aronow is attached to Westchester Medical Center, New York Medical College, New York.
Article History
Received: September 12, 2021 Accepted: October 21, 2021 Published: October 28, 2021
Citation: Das M, Aronow WS. Acute Myocardial Infarction in a Healthy 57-Year-Old Female; A Case Report. Int J. Hert & Vasclr Syst. 2021;2(1):24‒26. DOI: 10.51626/ijhvs.2021.01.00005
Abstract
Spontaneous coronary artery dissection (SCAD) commonly present as acute coronary syndrome in otherwise healthy population especially in women. SCAD is defined as dissection involving epicardial coronary arteries, not related to any trauma, or atherosclerosis process or any iatrogenic causes, resulting in intimal disruption and/or Intra-mural hematoma (IMH).
SCAD is often misdiagnosed and therefore the treatment is delayed resulting in complications. This review will focus on a case presentation and the diagnosis and management of SCAD.
Abbreviations
SCAD: Spontaneous Coronary Artery Dissection; FMD: Fibromuscular Dysplasia; IMH: Intramural Hematoma; STEMI: ST Elevation Myocardial Infarction; NSTEMI: Non-ST Elevation Myocardial Infarction; PCI: Percutaneous Coronary Intervention
Case Presentation
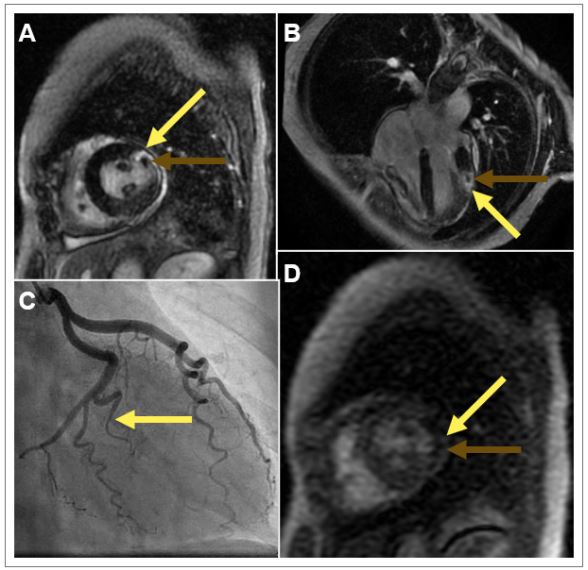
A 57-Year-old post-menopausal female presented to the emergency room with acute severe retrosternal chest pain, precipitated by severe emotional stress. The electrocardiogram showed normal sinus rhythm, incomplete right bundle branch block, diffuse T wave inversion inferior and anterior-lateral leads. Laboratory investigation showed elevated cardiac biomarkers. Echocardiogram showed normal left ventricular systolic function, no significant valvular abnormalities. Patient had a diagnostic coronary angiogram which showed spontaneous coronary artery dissection (SCAD) involving the obtuse marginal branches of the circumflex coronary artery (Figure 1C). Revascularization was deferred and patient was subsequently treated with medical management.
She continued to have chest pain and a cardiac MRI (Figure 1) showed preserved left and right ventricular systolic function, no significant valvular abnormalities, a resting perfusion defect in anterior-lateral wall segment of left ventricle (Figure 1D). A delayed gadolinium enhanced study showed an area of infarct in the obtuse marginal branch (of the circumflex coronary artery) (Figure 1A & Figure 1B). Her chest pain was presumed to be from partial reperfusion of small obtuse marginal branch artery dissection. She was advised to continue medical therapy and cardiac rehabilitation., Patient continued to do well and remain symptom free on her follow up.
Figure 1: Cardiac Magnetic Resonance Images and Coronary Angiogram.
A: Short axis section of the mid-ventricular cavity on T1 gadolinium enhanced imaging with transmural delayed gadolinium enhancement in the anterolateral wall (yellow arrow) and with evidence of microvascular obstruction (green arrow). B: Four (4) chamber T1 gadolinium enhanced section with the same findings as Box A. Yellow arrow indicating transmural infarction. Green arrow indicating microvascular obstruction. C: Right anterior oblique (RAO) caudal projection of the left coronary artery system revealing Type 1 spontaneous coronary artery dissection of the obtuse marginal 1 branch (yellow arrow). D: T1 perfusion imaging revealing a perfusion defect in mid anterolateral wall (yellow arrow).
Discussion
SCAD is most commonly presenting in female, commonly in age group 45-53 years and especially in pregnant and/or post-partum women. It is described in all races although it predominantly seen in whites [1,2]. SCAD mostly involve left anterior descending coronary artery (LAD) and less commonly involving other epicardial coronary arteries [3]. SCAD is often associated with fibromuscular dysplasia [4].
SCAD is presumed to result from spontaneous intramural hematoma (IMH) [5], mostly in the layers of tunica media, and compromising the true lumen, resulting in myocardial ischemia and infarction. SCAD often presents as chest pain and elevated cardiac biomarkers mimicking acute coronary syndrome: either STEMI or and NSTEMI [4], rarely as cardiogenic shock. Ventricular tachycardia and sudden cardiac death are less common presentation of SCAD [5,6].
A vulnerable patient and a potential trigger precipitates SCAD. Extreme physical stress (Intense isometric exercise or extreme weightlifting) and emotional stress (often due to catecholamine surge) and/or hormonal changes (related to pregnancy, post-partum or perimenopausal women), related to oral contraceptive or steroid use, are associated with this condition.
SCAD patients are often misdiagnosed or diagnosed late, resulting in delayed treatment. However early diagnosis is the key since the management. The management differs from contemporary atherosclerosis related myocardial infarction. Coronary angiogram (with or without intra-vascular ultrasound and optical coherence tomography) is the gold standard in diagnosis of SCAD [7]. Based on angiographic findings SCAD is classified in these following types [8-10].
Type I-Coronary angiogram showing radiolucent lumen with spiral dissection.
Type II-Most common type and coronary angiogram showing diffuse stenosis and distal tapering.
Type III -Least common type and coronary angiogram (and IVUS showing intramuscular hematoma) with a single or multiple focal stenosis mimicking atherosclerosis.
CT angiogram and cardiac MRA are also useful tools in diagnosis of SCAD- especially for diagnosis of fibromuscular dysplasia and atherosclerotic lesions in coronary artery.
Management
There is no randomized clinical trial (RCT) comparing early invasive treatment compared to medical management. The decision for treatment of SCAD patient depends on location and hemodynamic stability and presence of ischemia and/or chest pain. SCAD involving left main or proximal left anterior descending coronary artery are often treated by revascularization (PCI or CABG) [6]. SCAD in other coronary distribution is often managed conservatively. Routine use of intravenous heparin is avoided in patients who are treated conservatively to avoid the risk of extension of the intramuscular hematoma.
SCAD patients are treated with long-term daily aspirin and dual anti-platelet therapy post percutaneous coronary intervention (PCI). Beta-blockers are indicated SCAD patients with LV systolic dysfunction and /or with arrhythmia or in presence of hypertension. ACE I/ARB are indicated in patients who has other guideline directed indications for these medications- e.g., Left ventricular systolic dysfunction or presence of hypertension. SCAD patients are typically not treated with statin unless they are diagnosed to have hyperlipidemia. Antianginal therapy is indicated for patients presenting with recurrent chest pain with or without ischemia. Nitrates, calcium channel blocker and ranolazine are often used in these patients. All patients of SCAD should be referred for cardiac rehabilitation [6,11-15]. All SCAD patients should avoid any competitive sports and high intensity isometric exercise.
Conclusion
We describe a unique case of myocardial infarction in a post-menopausal woman related to SCAD. The management differs from contemporary atherosclerosis related myocardial infarction patients and avoiding revascularization unless absolutely indicated. Future studies are needed to decide optimum management in these unique patient population.
References
- Pretty HC (1931) Dissecting aneurysm of coronary artery in a woman aged 42. British Medical Journal 1: 667.
- Takahiro Nakashima, Teruo Noguchi, Seiichi Haruta, Yusuke Yamamoto, Shuichi Oshima, et al. (2016) Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol 207: 341-348.
- Sharonne N Hayes, Sridevi R Pitta, Robert D Simari, Amir Lerman, Ryan J Lennon, et al. (2012) Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 126(5): 579-588.
- Saw J, Poulter R, Fung A, Wood D, Hamburger J, et al. (2012) Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series Circ Cardiovasc Interv 5(1): 134-137.
- De Giorgio F, Abbate A, Vetrugno G, Capelli A, Arena V (2007) Non-atherosclerotic coronary pathology causing sudden death. Journal of Clinical Pathology 60(1): 94-97.
- Prakash R, Rajala J, Birnie T, Isserow S, Taylor CM, et al. (2016) The First Dedicated Cardiac Rehabilitation Program for Patients with Spontaneous Coronary Artery Dissection: Description and Initial Results. Can J Cardiol 32(4): 554-560.
- Rashid HN, Wong DT, Wijesekera H, Gutman SJ, Shanmugam VB, et al. (2016) Incidence and characterization of spontaneous coronary artery dissection as a cause of acute coronary syndrome–A single-centre Australian experience. Int J Cardiol 202: 336-338.
- Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, et al. (2012) Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 126(5): 579-588.
- Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, et al. (2012) 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 126(7): 875-910.
- Rogers JH, Lasala JM (2004) Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol 16(9): 493-499.
- Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, et al. (2016) Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol 207: 341-348.
- Jacqueline Saw (2014) Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 84(7): 1115-1122.
- Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, et al. (2012) Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 126(5): 579-588.
- Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, et al. (2017) Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol 70(9): 1148-1158.
- Krittanawong C, Tweet MS, Hayes SE, Bowman MJ, Gulati R, et al. (2016) Usefulness of Cardiac Rehabilitation After Spontaneous Coronary Artery Dissection. Am J Cardiol 117(10): 1604-1609.