Montmorillonite Clay Enhanced TiO2 Nanoparticle for Photocatalytic Degradation of Organic Pollutants: Mini Review
Kelechi E Onwuka1*, Kennedy Achilike2, Kalu S Eze1, Nnamdi E Enenwa1
1Department of Pure and Industrial Chemistry, Abia State University Uturu, Nigeria
2Department of Physics, Abia State University Uturu, Nigeria
*Corresponding author: Kelechi E Onwuka, Department of Pure and Industrial Chemistry, Abia State University Uturu, Nigeria
Article History
Received: April 14, 2021 Accepted: May 07, 2021 Published: May 10, 2021
Citation: Onwuka KE, Achilike K, Eze KS, et al. Montmorillonite Clay Enhanced TiO2 Nanoparticle for Photocatalytic Degradation of Organic Pollutants: Mini Review. Int J Pharma Sci. 2021;1(1):33‒38. DOI: 10.51626/ijps.2021.01.00004
Abstract
The presence of varieties of organic pollutants in the environment is a serious global concern. Improper disposal of industrial effluents as well as other anthropogenic activities accounts for the vast amount of organic pollutants present in the environment, consequently exposing living organisms to severe hazard. Photocatalysis can be applied to mitigate the presence of organic pollutants in aqueous solution. Regards to this, TiO2 has been highly studied as a phototcatalyst, although its limitations hampers its applicability. The limitations of TiO2 is a function of low surface area of about 50m2/g, porousity and the difficulty of separating it from a reaction mixture. To overcome these drawbacks various materials have been applied as catalytic support for TiO2 to enhance its phocatalytic activity. Among these, clays have been widely considered owing to their non-toxicity, low cost, availability, as well as their mechanical, chemical and thermal stability. Clays, especially montmorillonite shows high surface area, high availability of active sites and porousity. These attributes promises efficient support for TiO2 as a photocatalyst when supported on clas like montmorillonite (TiO2/Mt nanocomposite). The present review appraises different methods applied for the preparation of TiO2/Mt nanocomposite, impact of montmorillonite clay on physical and photocatalytic activity of TiO2, mechanism of TiO2 assisted photocatalytic degradation and applications of TiO2/Mt nanocomposite for photo degradation of organic pollutants.
Keywords: Photocatalytic degradation; TiO2/Mt; Organic pollutants; Montmorillonite; Mechanism
Introduction
The negative impact of organic pollutants in the environment can never be underestimated. Huge amount of effluents containing organic pollutants find their way into marine environments mainly originating from agricultural runoffs, industrial effluents, sewage plants and other anthropogenic activities [1]. Continuous breakdown of these organic compounds consumes considerable amounts of dissolved oxygen from the water bodies exceeding the rate of replenishment, leading to depletion of dissolved oxygen available for marine ecosystems. Organic compounds such as hydrocarbons (e.g. oil), aromatic compounds (e.g. phenols, biphenyls), polycyclic aromatic hydrocarbons (e.g. Naphthalene, phenanthrene, pyrene), pesticides, herbicides, pharmaceuticals, detergents, proteins and plasticizers are commonly found in water bodies [1]. In addition, numerous organic compounds found in wastewaters are non-biodegradable [1,2]. These pollutants especially pose significant concerns because they are persistent, high toxic compounds that are transported over long distances and bioaccumulated in the tissues of plants and aquatic organisms [3,4]. Also, a class of organic pollutants known as persistent organic pollutants (POPs) is largely released into water bodies from agricultural wastewaters, industrial effluents, waste incineration plants and metal production processes. These include organochlorine pesticides (e.g. dichloro-diphenyl- trichloroethane, dibenzo-p-dioxins, dibenzo-p-furans and hexachlorobenzene), and polychlorinated biphenyls, dibenzo-p-dioxins and dibenzofurans. Lastly, dyes form an important class of non-biodegradableorganic pollutants found in wastewaters [1]. Lastly, Dyes mainly originate from colored effluents from textile, pulp and paper industries. They are persistent carcinogenic compounds adversely affecting marine microbial and mammalian populations [1,2,5].
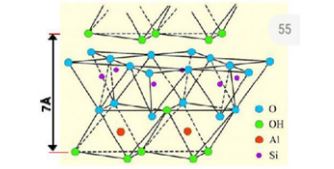
Clays are layered phyllosilicate minerals which occur naturally in the earth’s crust and are important constituents of soils [6-8]. Clay minerals show plasticity depending upon the water content and harden up when dried [9]. Clays possess extraordinary physiochemical properties such as cation exchange capacity, high surface reactivity, high adsorption capability as well as good swelling property and biocompatibility making them suitable for a wide variety of applications such as cosmetics, catalysis, pharmaceutics, medicine, and sensors [6]. Clay minerals comprise of tetrahedral (T) silica sheet and an octahedral sheet of either (O) gibbsite (Al(OH)3) or brucite (Mg(OH)2) stacked upon each other (Figure 1). Clay minerals are classified into two types: 1:1 and 2:1 depending upon the number of silica sheets stacked to either octahedral gibbsite or brucite 1:1 and 2:1 (Figure 2) [10]. The 1:1 clay consists of one tetrahedral silica sheet stacked to octahedral gibbsite or brucite sheet. Kaolinite is 1:1 clay consisting of tetrahedral silica and octahedral gibbsite sheet. Montmorillonite (Mt) on the other hand is 2:1 clay having two tetrahedral silica sheets and one octahedral gibbsite sheet. Isomorphic substitution of Al3+ for Si4+ in tetrahedral silica sheet and Mg2+ for Al3+ in octahedral sheet which gives rise to negative charge on clay surface which is balanced by the exchangeable cations in the interlayer space [6]. Clays can also be classified as either cationic or anionic based on their surface charge. Cationic clay minerals consist of negatively charged aluminosilicate surface and contain positively charged cations in their interlayer space to balance the surface charge and also posses interstitial water molecules [10,11]. On the other hand, anionic clays such as layered double hydroxides possess positively charged surface, are mostly synthetic and not occur as crude forms in nature [6,10].
Figure 1: Structure of tetrahedral and octahedral sheet forming (1:1) structure of kaolinite with a d-spacing of 7A (modified from Bergaya et al. [56]).
Figure 2 2:1 structure of smectites. The d-spacing varies from 10A to 20A depending on the amount of interlayer water.
Clays have been frequently applied as supports for TiO2. It has been found that clays tend to enhance the photocatalytic activity of TiO2 NPs. Pristine TiO2 NPs such as commercial Degussa P25 are less photoactive than clay supported TiO2. The enhancement of photocatalytic activity can be due to the high surface area, adsorption capability, porosity and presence of surface active sites in TiO2/clay nanocomposites [12]. The rise in photocatalytic activity is also due to lower charge recombination rate in TiO2/clay nanocomposites. The reduction in charge recombination in TiO2/clay nanocomposites by clay particles can be due to the presence of interlayer cations in clay which tend to trap electrons and let the holes free for oxidation [6,13]. Clay also enhances the reusable efficiency of TiO2 by making it separable from the reaction mixture. Clay minerals like kaolinite, smectite, montmorillonite (Mt), bentonite, palygorskite, halloysite, attapulgite, kunipia, rectorite, hectorite, laponite, diatomite and layered double hydroxides (LDH) have been utilized as TiO2 supports. For the preparation of TiO2/clay nanocomposites, impregnation of TiO2 either on clay surface or between its layers is highly preferred. For this purpose titanium (IV) alkoxides such as titanium isopropoxide and titanium (IV), butoxides and low-cost titanyl sulphate (TiOSO4) and titanium tetrachloride (TiCl4) are frequently and commonly applied as starting materials. Therefore the present review focuses upon the photocatalytic application of TiO2/Montmorillonite clay nanocomposite for degradation of organic pollutants, however there are some reports explaining the applications such as packaging [6,14], CO2 reduction [13], sunscreens [15], antibacterial activities, etc. However, some clays depicts pozzolanic and cement like attributes, so the corresponding nanocomposites have highly promising applications in building materials. Hence, it can be said that the TiO2/Montmorillonite nanocomposite have positive future perspective for day to day application, as well as industrial usage. This review emphasize on the properties, preparation methods as well as photoactivity of TiO2/Montmorillonite nanocomposites.
Mechanism of TiO2-Assisted Photocatalytic Degradation
Detailed reports on the mechanism of TiO2-assisted photocatalysis is already available in literature [16-24]. Photocatalytic reaction is initiated when an aqueous dispersion of TiO2 is illuminated with light energy greater than its band gap energy (e.g., 3.2eV) and conduction band electrons (e−) are generated. Consequently, valence band holes (h+) are generated (Eq. (1). The photogenerated holes can react with OH– or H2O oxidizing them into OH• radicals (Eqs. (2)–(3). Since oxygen is an easily reducible substance, the reduction of oxygen adsorbed on the Ti(III)-surface or dissolved in water by the photoelectron of the conduction band results in generating superoxide radical anions (O2•−), which in turn react with H+ to generate hydrogen dioxide radical (•HO2, hydroperoxyl) (Eqs. (5)–(7)). During subsequent collisions with an electron a hydrogendioxide (1-) anion (HO2−,hydrogenperoxide(1) is produced and H2O2 is eventually formed (Eqs. (7)–(11).
TiO2 + hv → TiO2 (h+ + e-) (1)
Reaction involving valence band h+
TiO2 (h+) + H2O→TiO2 + •OH + H+ (2)
TiO2 (h+) + OH–→TiO2 + •OH (3)
TiO2 (h+) + 2H2O →TiO2 + H2O2 + 2H+ (4)
Reaction involving conduction band e−
TiO2(e−) + O2→TiO2 + O2•− (5)
O2•− + H+→HO2• (6)
TiO2(e−) + HO2• →TiO2 + HO2− (7)
TiO2(e−) + O2• + 2H+ →TiO2 + H2O2 (8)
TiO2(e−) + H2O2 → TiO2 + •OH + OH− (9)
O2•− + H2O2→ •OH + OH− + O2 (10)
2HO2• →O2 + H2O2 (11)
Photoholes have great potential to oxidize organic species directly or indirectly via the combination with •OH predominant in aqueous solutions (Eqs. (12) and (13) [16,19].
R-H + •OH→R`• + H2O (12)
R-H + h+ →R+•→Degradation products (13)
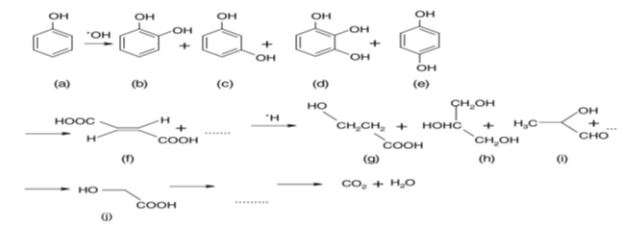
Hydroxyl radicals (•OH), holes (h+), superoxide ions (O2•−) and peroxide radicals (•HO2) are highly reactive intermediates that will oxidize a large variety of organic compounds [16,25-27]. Photooxidative degradation of many organic pollutants over titania has been extensively reviewed [28,29]. Both the mechanism of decomposition and the number of intermediates depend upon the nature of organic compounds. The degradation of phenol, aniline and its derivatives has been widely studied [16,26,30-34], owing to the toxicity, great persistency and low natural biodegradability of these compounds. During the photochemical degradation of phenol with TiO2 the OH• radical attacks the phenyl ring of the phenol molecule, giving rise to several intermediates [16,34]: catechol (b), resorcinol (c), benzene-1,2,3-triol(d) and hydroquinone (e), then the phenyl rings in these compounds break up to give maleic acid (f), then short-chain organic acids such as, 3-hydroxy propyl carboxylic acid (g), 2-hydroxy propanal (i), 2-hydroxy-ethanoic acid glycol acid (j), finally CO2 and H2O (Figure 3).
Figure 3: Phenol photodegradation route in aqueous solution over TiO2[34].
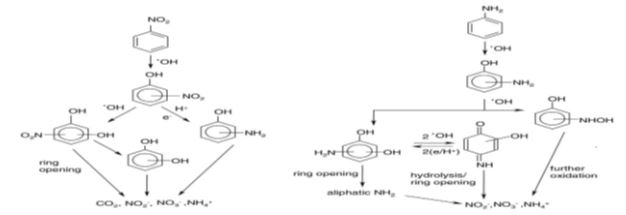
The photocatalytic degradation of 2-, 3- and 4-nitrophenol in oxygenated aqueous dispersions containing TiO2 leads to complete mineralisation of the substrates [31,35]. The formation of dihydroxynitrobenzene isomers confirms that the hydroxyl radical oxidation is the major reaction pathway in the photocatalytic degradation of the three isomeric nitrophenols. Nitrite ions are formed in the early stages of the process, whereas nitrate and ammonium ions are present at the end of the degradation (Figure 4). As intermediate products resulting from the photodegradation of aniline, some hydroxylated aromatic compounds such as phenol, 2-aminophenol, hydroxyhydroquinone, paraquinone and nitrobenzene were formed [26,30,35]. The formation of these intermediates confirmed the •OH radical mediated mechanism of aniline photodegradation (Figure 4).
Figure 4 Decomposition pathways for aniline and nitrobenzene in aqueous solution over TiO2[35].
TiO2/Mt Nanocomposites
Numerous studies have reported the efficacy of TiO2/Montmorillonite nanocomposite for photodegradation of organic pollutants. Ding et al. [36] synthesized TiO2 pillared Mt nanocomposites using sol-gel method accompanied by different drying processes such as air drying, ethanol extraction drying and supercritical drying. It was observed that drying processes had significant impacts upon their photocatalytic activities against phenol degradation. The nanocomposite obtained by supercritical drying process showed high photoactivity as a result of high surface area and crystallinity of TiO2. In another study, Ooka et al. [37] prepared TiO2 pillared Mt nanocomposites by the hydrothermal method which led to the formation of highly crystalline anatase TiO2 in the size range 40-60Å. The as-synthesized nanocomposites had high photocatalytic activity regarding trichloroethylene degradation in water. Jagtap et al. [38] carried out pillaring of Mt with TiO2 via conventional stirring and ultrasonic agitation accompanied by hydrothermal treatment. Ultrasonic treatment resulted in the fast formation of TiO2 pillared Mt nanocomposite within 20 minutes. Nanocomposites synthesized d by ultrasonic as well as conventional stirring methods had TiO2 in both anatase and rutile phase. However, the rutile phase was highly prominent in nanocomposites prepared by conventional stirring. The rutile content also increased upon increasing the duration of hydrothermal treatment. The nanocomposites were then employed for degradation of aniline which was selectively oxidized to azoxybenzene at ambient temperature [6].
Nanocomposites prepared in shorter time by ultrasonic treatment were highly active in aniline photo-oxidation. TiO2 pillared Mt was also prepared by the solvothermal process using ethanol and water as solvents, hexamethylenetetramine as precipitant and TiCl3 as a precursor. The resultant nanocomposite displayed the mesoporous structure with pore diameters 6-10nm and optimized surface area. The photocatalytic activity was investigated by degradation of methylene blue (MB) dye in the aqueous medium and it was found to be dependent upon the ratio of TiCl3 and Mt. The nanocomposite by taking TiCl3 and Mt in the ratio 0.2:1 displayed the highest photoactivity due to high surface area and porosity [39]. Djellabi et al. [40] synthesized TiO2/Mt nanocomposites via impregnation of TiCl4 on Mt accompanied by calcination at 350oC which resulted in the formation of crystalline anatase TiO2 on clay surface. TiO2/ pillared Mt nanocomposites were prepared at lower temperatures (30-80oC) and without calcination by Zhang et al. [41].
Pillaring of TiO2 on Mt was carried out by impregnating TiO2 sol synthesized form low cost TiCl4 between layers of Mt. Pillaring of TiO2 interfered greatly with the layered structure of Mt and led to the formation of TiO2 nanoparticles (TiO2 NPs) on the surface and in inter-layer space of Mt. The photoactivity was carried out under UV-A radiation and was evaluated with respect to the degradation of five cationic dyes such as MB , RhB, Methyl orange (MO), crystal violet and congo red. The degradation rates were found to be in the order: crystal violet (97.1%) > MB (93.2%), RhB (79.8%) > MO (36.1%) > congo red (22.6%). From the comparative study it is evident that the photoactivity depended upon the contact between the TiO2 NPs on Mt surface and dye molecules.
Apart from above mentioned methods, the intercalation of TiO2 NPs on Mt can also be performed by using long chain surfactants such as polyoxyproplylene (POP), Hexadecyltrimethyl ammonium bromide (HDTMA) etc. The colloidal TiO2 particles were intercalated into Mt layers by applying POP as expanding agent to synthesize TiO2 pillared Mt nanocomposites by Chen et al. [42]. The introduction of POP not only led to the formation of delaminated structure [6] but also incremented the surface area and porosity of the nanocomposite. Photocatalytic degradation of methylene blue dye by these nanocomposites implied that the surface area is a crucial parameter influencing the photoactivity of the nanocomposites due to increased contact between dye and catalyst. Chen et al. [43] used CTAB as expanding agent to intercalate Ti alkoxide precursor between Mt layers. Here, CTAB played a similar role as that of POP discussed above in increasing the surface area and porosity of the nanocomposites. It led to the homogeneous distribution of TiO2 NPs on Mt. The intercalation of Ti precursor led to the formation of anatase TiO2 NPs in the size range 5-10nm. The nanocomposite was found to possess great thermal stability since there was no phase transformation from anatase to rutile even after calcination at 900oC. The photocatalytic activity was investigated by methylene blue degradation and nanocomposites exhibited better photoactivity than commercial P25 with maximum degradation efficiency up to 99% within 60 minutes. Similarly, Dvininov et al. [44] reported the use of cetyl trimethyl cations (CTA+) for expanding Mt layers so as to adsorb titanium alkoxide precursors between them under acidic conditions. TiO2 was obtained by subsequent calcination of titanium alkoxide. The size of TiO2 pillars was directly proportional to the size of Ti-polycationic species interlayer space. The photoactivity of the nanocomposites was investigated by degrading congo red dye under UV light. The photoactivity was highly dependent upon TiO2 pillar size and increase in pillar size resulted to enhanced contact between the dye molecules and photocatalyst leading to high photoactivity. Other than these techniques, there are some other techniques developed to impregnate TiO2 between Mt interlayers such as hetero-coagulation [6] and pH controlled hydrothermal process [45]. Mogyorosi et al. [46] synthesized TiO2/Mt nanocomposites by two distinct methods. In the first method, the titanium alkoxides were adsorbed on to Mt and subsequently hydrolyzed to TiO2. In the second method hetero-coagulation of TiO2 NPs between Mt interlayer spaces was carried out. In both these processes, the TiO2 NPs were found to successfully intercalate between Mt layers. Highly crystalline anatase TiO2 NPs of size less than 5nm were found to exist in the nanocomposites prepared by both the methods. The nanocomposites prepared by hetero-coagulation method were found to exhibit high surface area. Kun et al. [47] employed Hetero-coagulation method in preparing TiO2/Mt nanocomposites under highly acidic (pH~1) and weakly acidic conditions (pH~4). Pure anatase phase of TiO2 was obtained in the nanocomposites and TiO2 content was varied from 20 to 75%. The samples synthesized under highly acidic conditions displayed high surface areas ranging from 171-284m2/g. The photocatalytic activity of the nanocomposites was investigated by oxidation of phenol which enhanced upon intercalation of TiO2 in Mt interlayer spaces. Recently, the self assembling of Mt microlayers was carried to establish a sandwiched layered structure of TiO2 intercalated on Mt by Huo et al. [45]. The synthesis was carried out under pH controlled hydrothermal process. The size of intercalated TiO2 was around 15nm. The nanocomposite displayed much higher photocatalytic activity and recycling ability regarding the degradation of MO dye occasioned by generation of Ti3+ active sites and high surface area and pore volume. The nanocomposite also countered the limitations such as limited mass transfer efficiency and light barrier.
Other than enhanced surface area and porosity immobilization of Fe3+ ions on nanocomposites can result to faster and efficient photocatalysis as a result of combined Fenton and photocatalytic oxidation as reported by Munawwarah et al. [48]. Further investigation on the role of Fe species loading of Fe on TiO2/Mt nanocomposites were carried out by Okte et al. [49] via in-situ growth of TiO2 and Fe species on Mt surface. Presence of mixed valence of Fe played a crucial role in electron transfer processes which enhanced the photocatalytic activities of Mt supported TiO2 regarding β-Naphthol degradation. The nanocomposites had higher surface area, extended optical absorption profiles through longer wavelengths and pore volume. Owing to their mesoporousity, the nanocomposites had better adsorption and degradation capabilities [6]. The Fe species present in the nanocomposites mediated the electron transfer processes during photocatalytic reactions. The degradation kinetics of β-Naphthol followed the Langmuir- Hinshelwood mechanism. Although, TiO2/clay nanocomposites can be easily separated from the reaction mixture and have high reusability compared to commercial P25, but complete separation is still a challenge as some amount of the catalyst is lost during commonly used separation processes such as decantation, centrifugation, filtration etc. In order to overcome this limitation magnetically separable nanocomposites can be a highly promising strategy to obtain much higher reusability. In this regard, TiO2/Mt/Fe3O4 nanocomposites were synthesized by hydrolysis of Fe3O4-tetra-nbutyl titanate micro-emulsion to form Fe3O4 loaded TiO2 NPs in the interlayer space of Mt [50]. The size of an obtained TiO2 NPs were found to be in the range of 10-20nm whereas size of Fe3O4 NPs were in the range of 40-60nm. The nanocomposite was able to degrade about 94% of MB dye in comparison to 85% by pristine Ti NPs. The nanocomposite retained high photoactivity even after being used for six consecutive runs which exposes its high reusable efficiency.
Although TiO2/Mt nanocomposites likely dipicts high photoactivity compared to commercial and unsupported TiO2, it is inactive in the visible region of solar spectrum owing to wide band gap of TiO2 (3.2eV). In order to enhance its activity in the visible light, several attempts have been made which are almost similar to those of TiO2 alone. Liu et al. [51] synthesized silver (Ag) metal loaded TiO2/Mt nanocomposites by hydrolysis of TiCl4 between Mt interlayer spaces and continuously loading AgNPs by reduction of silver nitrate. According to their report, silver loading enhanced the light absorption of nanocomposites as a result of LSPR effect which led to high photocatalytic activity of Ag-TiO2/Mt when compared to TiO2/Mt and commercial P25 under UV light. Elemental doping can be another option for obtaining visible light active nanocomposite. For this purpose, Zhang et al. [41,52] have prepared nitrogen and sulphur co-doped TiO2/Mt nanocomposites by impregnating doped-TiO2 sol into layers of Mt. The doping of nitrogen and sulphur led to red shift in the absorption edge of UV-Visible diffuse reflectance spectra of TiO2/Mt nanocomposites. The nanocomposites were found to successfully degrade 4 BS and Acid red-G dye under visible light irradiation. Solophenyl-3BL dye was degraded by TiO2/pillared Mt prepared by microwave synthesis under both visible and UV light irradiation [53]. It was proposed that the electron transfer from excited dye molecule to conduction band of TiO2 resulted in photosensitization of TiO2. However, the process represented about 25% of total photoactivity. It was also found by total organic carbon (TOC) analysis that only 6% of the dye was photo-mineralized under visible light illumination with a low rate constant. Carbon (C) and Vanadium (V) doped TiO2/Mt nanocomposites were synthesized by Chen et al. [54,55] and were employed for degradation of sulphorhodamine-B dye under UV as well as visible light. The photocatalytic activities of V-TiO2/Mt and C-V-TiO2/Mt were higher than that of V-TiO2 and C-V-TiO2 in both UV and visible light. However, C-TiO2/Mt nanocomposite had lower photoactivity than C-TiO2. The C-TiO2 had higher photoactivity under both UV and visible light than V-TiO2 and C-V-TiO2 which reveals elemental carbon to be a better photo-sensitizer. However, low photoactivity of C-TiO2/Mt than C-TiO2 was attributed to the fact that Mt mediated the transfer of electrons from excited carbon species to vacant d orbital of transition metals present on Mt and slowed down the photoactivity of C-TiO2/Mt.
Conclusion
This review emphasizes on the properties of TiO¬2/Montmorillonite nanocomposites and the role of montmorillonite clay as a supports on photocatalytic activity of TiO2 NPs for the photodegradation of organic compound. It also presents the properties of montmorillonite supported heterostructured TiO2 photocatalysts. Montmorillonite clay plays crucial role in photocatalytic activity. Montmorillonite clay minerals has been applied for TiO2/Mt nanocomposite synthesis discussed in the current review. TiCl4, Titanium (IV) butoxide, tetrabutyl titanate and titanium isopropoxide are commonly applied starting materials for TiO2 nanocomposite preparation. Numerous methods have been developed for the preparation of TiO2/Mt nanocomposites such as: hydrothermal, solvothermal, microwave assisted, ultrasonic, heterocoagulation and sol-gel, etc. The application of different surfactant has promoted the photocatalytic activity of nanocomposites owing to increment in specific surface area and porosity. Pore size, pore volume and interlayer space of clay minerals can vary by the utilization of surfactants such as HDTMA (CTAB), POP. The variation in interlayer space, pore volume and pore size is a function of concentration and surfactant chain length. Therefore TiO2/Mt nanocomposite promises to be a very effective photocatalyst for photodegradation of organic pollutants.
References
- Awad AM, Shaikh SMR, Jalab R, Gulied MH, Nasser MS, et al. (2019) Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Separation and Purification Technology 228: 115719.
- Rashed MN (2013) Adsorption technique for the removal of organic pollutants from water and wastewater. In: MN Rashed (Ed.), Organic Pollutants – Monitoring, Risk and Treatment. Intech Open: Croatia; 2013: 167–179.
- Abbas M, Adil M, Ehtisham-ul-haque S, Munir B, Yameen M, et al. (2018) Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment : A review. Sci Total Environ 626: 1295–1309.
- Iqbal M, Abbas M, Nisar J, Nazir A, Qamar AZ (2019) Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: A review. Chem Int 5(1): 1–80.
- Chham A, Khouya EH, Oumam M, Abourriche A, Gmouh S (2018) The use of insoluble mater of Moroccan oil shale for removal of dyes from aqueous solution: The use of insoluble mater of Moroccan oil shale for removal of dyes from aqueous solution. Chem Int 4(1): 67–76.
- Mishra A, Mehta A, Basu S (2018) Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A Review. Journal of Environmental Chemical Engineering 6(5): 6088-6107.
- Mueller B (2015) Experimental interactions between clay minerals and bacteria: A review. Pedosphere 25: 799-810.
- Gomes CDSF, Silva JBP (2007) Minerals and clay minerals in medical geology. Applied Clay Science 36(1-): 4-21.
- Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chemical Engineering Journal. 308: 438-462.
- Ghadiri M, Chrzanowski W, Rohanizadeh R (2015) Biomedical applications of cationic clay minerals. Rsc Advances 5(37): 29467-29481.
- Reichle WT (1986) Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ionics 22(1): 135-141.
- Manova E, Aranda P, Martín-Luengo MA, Letaïef S, Ruiz-Hitzky E (2010) New titania-clay nanostructured porous materials. Microporous and Mesoporous Materials 131(1-3): 252-260.
- Tahir M, Amin NS (2013) Photocatalytic reduction of carbon dioxide with water vapors over montmorillonite modified TiO2 nanocomposites. Applied Catalysis B: Environmental 142: 512-522.
- Paiva JP, Santos BA, Kibwila DM, Gonçalves TC, Pinto AV, et al. (2014) Titanium Dioxide Montmorillonite Nanocomposite as Photoprotective Agent Against Ultraviolet B Radiation‐Induced Mutagenesis in Saccharomyces cerevisiae: A Potential Candidate for Safer Sunscreens. Journal of pharmaceutical sciences 103(8): 2539-2545.
- Moghaddam HM, Khoshtaghaza M, Salimi A, Barzegar M (2014) The TiO2–Clay-LDPE nanocomposite packaging films: investigation on the structure and physicomechanical properties. Polymer-Plastics Technology and Engineering 53(17): 1759-1767.
- Szczepanik B (2017) Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Applied Clay Science 141: 227–239.
- Zhan H, Tian H (1998) Photocatalytic degradation of acid azo dyes in aqueous TiO2 suspension I. The effect of substituents. Dyes and Pigments 37(3): 231–239.
- Bandara J, Mielczarski JA, Kiwi J (1999) 2. Photosensitized degradation of azo dyes on Fe, Ti and Al oxides. Mechanism of charge transfer during the degradation. Langmuir 15: 7680–7687.
- Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol C: Photochem Rev 1(1): 1–21.
- Tanaka K, Padermpole K, Hisanaga T (2000) Photocatalytic degradation of commercial azo dyes. Water Research 34: 327–333.
- Bianco-Prevot A, Baiocchi C, Brussino MC, Pramauro E, Savarino P, et al. (2001) Photocatalytic degradation of Acid Blue 80 in aqueous solutions containing TiO2 suspensions. Environ Sci Technol 35(5): 971–976.
- Galindo C, Jacques P, Kalt A (2001) Photochemical and photocatalytic degradation of an indigoid dye: a case study of Acid Blue 74 (AB74). Journal of Photochemistry and Photobiology A: Chemistry 141(1): 47–56.
- Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, et al. (2001) Photocatalytic degradation pathway of methylene blue in water. Applied Catalysis B: Environmental 31(2): 145–157.
- Daneshvar N, Salari D, Khataee AR (2003) Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. Journal of Photochemistry and Photobiology A: Chemistry 157(1): 111–116.
- Kumar A, Mathur N (2004) Photocatalytic oxidation of aniline using Ag-loaded TiO2 suspensions. Applied Catalysis A: General 275(1-2): 189–197.
- Canle LM, Santaballa JA, Vulliet E (2005) On the mechanism of TiO2-photocatalyzed degradation of aniline derivatives. Journal of Photochemistry and Photobiology A: Chemistry 175(2-3): 192–200.
- Karunakaran C, Senthilvelan S, Karuthapandian S (2005) TiO2-photocatalyzed oxidation of aniline. Journal of Photochemistry and Photobiology A: Chemistry 172(2): 207–213.
- Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 9(1): 1–12.
- Sanchez L, Peral J, Domenech X (1997) Photocatalyzed destruction of aniline in UV-illuminated aqueous TiO2 suspensions. Electrochima Acta 42(12): 1877–1882.
- Di Paola A, Augugliaro,V, Palmisano L, Pantaleo G, Savinov E (2003) Heterogeneous photocatalytic degradation of nitrophenols. Journal of Photochemistry and Photobiology A: Chemistry 155(1-3): 207–214.
- Chu W, Choy WK, So TY (2007) The effect of solution pH and peroxide in the TiO2-induced photocatalysis of chlorinated aniline. J Hazard Mater 141(1): 86–91.
- Silva AMT, Silva CG, Dražić G, Faria JL (2009). Ce-doped TiO2 for photocatalytic degradation of chlorophenol. Catalysis Today 144(1-2): 13–18.
- Guo Z, Ma R, Li G (2006) Degradation of phenol by nanomaterial TiO2 in wastewater. Chemical Engineering Journal 119(1): 55–59.
- Carp O, Huisman CL, Reller A (2004). Photoinduced reactivity of titanium dioxide. Progress in Solid State Chemistry 32(1-2): 33–177.
- Ding Z, Zhu H, Lu G, Greenfield P (1999) Photocatalytic properties of titania pillared clays by different drying methods. Journal of Colloid and Interface Science 209(1): 193-199.
- Ooka C, Akita S, Ohashi Y, Horiuchi T, Suzuki K, et al. (1999) Crystallization of hydrothermally treated TiO2 pillars in pillared montmorillonite for improvement of the photocatalytic activity. Journal of Materials Chemistry 9(11): 2943-2952.
- Jagtap N, Ramaswamy V (2006) Oxidation of aniline over titania pillared montmorillonite clays. Applied Clay Science 33(2): 89-98.
- Liu J, Dong M, Zuo S, Yu Y (2009) Solvothermal preparation of TiO2/montmorillonite and photocatalytic activity. Applied Clay Science 43(2): 156-159.
- Zhang GK, Ding XM, He FS, Yu XY, Zhou J, et al. (2008) Low-temperature synthesis and photocatalytic activity of TiO2 pillared montmorillonite. Langmuir 24(3): 1026-1030.
- Djellabi R, Ghorab M, Cerrato G, Morandi S, Gatto S, et al. (2014) Photoactive TiO2–montmorillonite composite for degradation of organic dyes in water. Journal of Photochemistry and Photobiology A: Chemistry 295: 57-63.
- Chen D, Zhu Q, Zhou F, Deng X, Li F (2012) Synthesis and photocatalytic performances of the TiO2 pillared montmorillonite. J Hazard Mater 235: 186-193.
- Chen D, Du G, Zhu Q, Zhou F (2013) Synthesis and characterization of TiO2 pillared montmorillonites: application for methylene blue degradation. Journal of colloid and interface science 409: 151-157.
- Dvininov E, Popovici E, Pode R, Cocheci L, Barvinschi P, et al. (2009) Synthesis and characterization of TiO2-pillared Romanian clay and their application for azoic dyes photodegradation. Journal of hazardous materials 167(1-3): 1050-1056.
- Mogyorosi K, Dekany I, Fendler J (2003) Preparation and characterization of clay mineral intercalated titanium dioxide nanoparticles. Langmuir 19: 2938-2946.
- Huo M, Guo H, Jiang Y, Ju H, Xue B, et al. (2018) A facile method of preparing sandwich layered TiO2 in between montmorillonite sheets and its enhanced UV-light photocatalytic activity. Journal of Photochemistry and Photobiology A: Chemistry 358: 121-129.
- Kun R, Mogyorósi K, Dékány I (2006) Synthesis and structural and photocatalytic properties of TiO2/montmorillonite nanocomposites. Applied Clay Science 32(1-2): 99-110.
- Al-Munawwarah AM, Arabia S (2009) Combined Photocatalytie and Fenton Oxidation of Methyl Orange Dye using Iron Exchanged Titanium Pillared Montmorillonite. Journal of Applied Sciences 9(20): 3715-3722.
- Ökte AN, Tuncel D, Pekcan AH, Özden T (2014) Characteristics of iron‐loaded TiO2‐supported montmorillonite catalysts: β‐Naphthol degradation under UV‐A irradiation. Journal of Chemical Technology and Biotechnology 89(8): 1155-1167.
- Zhang P, Mo Z, Han L, Zhu X, Wang B, et al. (2014) Preparation and photocatalytic performance of magnetic Tio2/montmorillonite/Fe3O4 nanocomposites. Ind Eng Chem Res 53(19): 8057-8061.
- Liu J, Li X, Zuo S, Yu Y (2007) Preparation and photocatalytic activity of silver and TiO2 nanoparticles/montmorillonite composites. Applied Clay Science 37(3-4): 275-280.
- Zhang G, Ding X, He F, Yu X, Zhou J, Hu Y, Xie J (2008) Preparation and photocatalytic properties of TiO2–montmorillonite doped with nitrogen and sulfur. Journal of Physics and Chemistry of Solids 69(5-6): 1102-1106.
- Zhang G, Ding X, Hu Y, Huang B, Zhang X, et al. (2008) Photocatalytic degradation of 4BS dye by N, S-codoped TiO2 pillared montmorillonite photocatalysts under visible-light irradiation. The Journal of Physical Chemistry C 112(46): 17994-17997.
- Damardji B, Khalaf H, Duclaux L, David B (2009) Preparation of TiO2-pillared montmorillonite a photocatalyst Part I. Microwave calcination, characterisation, and adsorption of a textile azo dye. Applied Clay Science 44(3-4): 201-205.
- Chen K, Li J, Li J, Zhang Y, Wang W (2010) Synthesis and characterization of TiO2–montmorillonites doped with vanadium and/or carbon and their application for the photodegradation of sulphorhodamine B under UV–vis irradiation. Colloids and Surfaces A: Physicochemical and Engineering Aspects 360(1-3): 47-56.
- Chen K, Li J, Wang W, Zhang Y, Wang X, et al. (2011) The preparation of vanadium-doped TiO2– montmorillonite nanocomposites and the photodegradation of sulforhodamine B under visible light irradiation. Applied Surface Science 257(16): 7276-7285.
- Bergaya F, Theng BKG, And Lagaly G (2006) Surface area and porosity. In: Handbook of Clay science. Elsevier: Amsterdam; Netherlands: pp. 965.