Berberine Shows Proapoptotic Effect and Potentiation of Cytotoxic Drugs Acitivity in Colorectal Adenocarcinoma Cells
Martina Bago Pilatova*, Marek Sarissky
Department of Pharmacology, Faculty of Medicine, P J Safarik University, Kosice, Slovak Republic
*Corresponding author: Martina Bago Pilatova, Department of Pharmacology, Faculty of Medicine, Pavol Jozef Safarik University, Slovak Republic
Article History
Received: July 25, 2021 Accepted: July 30, 2021 Published: August 23, 2021
Citation: Pilatova MB, Sarissky M. Berberine Shows Proapoptotic Effect and Potentiation of Cytotoxic Drugs Acitivity in Colorectal Adenocarcinoma Cells. Int J Onco Radiother. 2021;2(1):22‒26. DOI: 10.51626/ijor.2021.02.00006
Abstract
Colorectal cancer, despite advances in treatment and diagnosis, is one of the most common causes of death from cancer. Conventional anticancer treatment is associated with serious side effects, therefore, new strategies are needed to reduce its toxicity. Natural compound berberine has shown antiproliferative activity against various cancer cells including colon. To investigate antiproliferative effect of berberine in human colorectal adenocarcinoma cells and its ability to potentiate the effect of convenient cytostatics taxol, docetaxel, doxorubicin and cisplatin we used MTT assay and flow cytometry was used to detect its proapoptotic effect and effect on cell cycle. In our experiments we observed significant antiproliferative activity of berberine chloride with IC50 = 6.7526 μM. Combination of berberine chloride (1μM) with taxol, docetaxel, doxorubicin and carboplatin significantly increased their antiproliferative activity and decreased their IC50 values. Cell cycle analysis revealed increase in sub-G0/G1 fraction considered as apoptotic and very mild increase in G0/G1 fraction in berberine chloride (5μM) treated cells. proapoptotic effect of berberine chloride (5μM) was confirmed by Annexin V/PI staining. Based on our results, we can assume that berberine has antiproliferative effect and the combination of berberine with cytostatics could be a promising alternative treatment for cancer. Further studies are necesary to investigate the exact mechanisms of action and drug – drug interactions.
Keywords: Berberine; Natural; Cancer; Antiproliferative; Apoptosis; Taxol; Docetaxel; Doxorubicin; Carboplatin; Zlom strany
Introduction
Colorectal cancer (CRC) is the third most common type of cancer after lung and breast cancer, and is the fourth most common cause of death worldwide from these diseases [1]. Conventional anticancer treatment is very effective in some patients, but is associated with high toxicity, which leads to serious side effects that reduce the patient’s quality of life. This fact, as well as efforts to increase the effectiveness of cancer treatment, is leading scientific teams to look for new compounds with lower toxicity and a better therapeutic response.
Natural compounds play important role in preventing cancer. In past few decades there has been a rise in the search after plant-derived drugs, the NCI (National Cancer Institute) has screened 35 thousand plant samples against numerous tumor types and the result is that 62% of the approved anticancer drugs appeared to be of natural origin. Some examples are vincristine and vinblastine (Catharanthus roseus), paclitaxil (T.brevifolia, T.candidiasis), amptothecin (Camptotheca acuminata), elliptinium (Bleekeria vitensis), ipomeanol (Ipomoea batatas) [2,3].
Berberine, a quaternary isoquinoline alkaloid, occurs in herbs such as Berberis aristata [4,5], Berberis darwinii [6,7], Berberis petiolaris [8] Berberis vulgaris [9] and Chinese goldthread (Coptis chinensis) [10] and goldenseal (Hydrastis canadensis) [11,12]. The diversity of berberine’s effect including antibacterial [13], anti-inflammatory [14] antidiabetic [15], antiobesity [16] and antidementia [17] effects have already been documented. In traditional Chinese medicine it is used for treatment of gastrointestinal complaints, such as diarrhea and dysentery [18]. Antiproliferative activity of berberine was observed against various cancer cells including cervical, colon, esophageal, gastric, prostate, epidermoid, melanoma, glioblasoma cancer cells and leukemia. It should be noted that the antitumor effect may vary depending on the type of cancer cells, the duration of treatment and the dose of berberine. Studies dealing with the potential mechanism of the antitumor effect of berberine have shown its ability to induce apoptosis, cell cycle arrest as well as affect genes involved in these processes, but it is not fully known how berberine initiates a cascade leading to them [19-26]. Bao J. et al. [27] describes hormetic (biphasic) effect of berberine which at low concentrations (1.25 ~ 5 μM) promoted cell proliferation while at high concentrations (10 ~ 80 μM) showed antiproliferative effect and concomitant treatment with low doses of berberine with conventional cytostatics fluorouracil (5-FU), camptothecin (CPT), and paclitaxel (TAX) attenuates their effect [27].
In this study, we investigated antiproliferative and proapoptotic effect of berberine in human colorectal adenocarcinoma cells and the effect of berberine at ineffective concentration in combination with convenient cytostatics taxol, docetaxel, doxorubicin and cisplatin.
Materials and Methods
Reagents
MTT (3-(dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was from Sigma – Aldrich Chemie (Steinheim, Germany). Tested berberine chloride was purchased from Sigma – Aldrich Chemie (Steinheim, Germany). Cycle TESTTM PLUS DNA Reagent Kit, annexin V-FITC and propidium iodide were purchased from Becton Dickinson (Becton-Dickinson, San Jose, CA, USA).
Cell culture
Caco-2 (human colorectal adenocarcinoma) were maintained in high glucose Dulbecco’s Modified Eagle Medium (GE Healthcare, Piscataway, NJ, USA), supplemented in with 10% foetal calf serum, penicillin (100 IU/ml) and streptomycin (100 g/ml) (all from Invitrogen, Carlsbad, CA, USA), in the atmosphere 5% CO2 in humidified air at 37oC. Cell viability, estimated by trypan blue exclusion, was greater than 95% before each experiment.
Assessment of cytotoxicity and potentiation of cytotoxic drugs activity by MTT assay
Potentiation of cytotoxic drugs activity was evaluated The cytotoxic effect of berberine chloride was studied by using colorimetric assay with the MTT end-point. The measured amount of MTT reduced to formazan was proportional to the number of viable cells [28]. Briefly, 5 × 103 cells were plated per well in 96-well polystyrene microplates (Sarstedt, Germany) in the culture medium containing berberine at final concentrations 100 – 0.1 μM. The MTT assay was also used to assess the effect of a combination of conventional cytostatics taxol: concentrations: 50, 12.5, 3.125, 0.78 μg/mL; docetaxel: concentrations: 50, 12.5, 3.125, 0.78 μg/mL; doxorubicin: concentrations: 2, 0.5, 6.25, 1.56 μg/mL; carboplatin: concentrations: 100, 25, 3.125, 0.78 μg/mL and berberine at ineffective concentration 1 μM. The concentrations of cytostatics used in our experiment corresponded to the concentrations used to measure chemosensitivity of tumor cells obtained from patient samples. After 72 h, 10 l of MTT (5 mg/mL) were added in each well. After an additional 4 h 100 L of 10% sodium dodecylsulphate were added in each well and another 12 h were allowed for the formazan to be dissolved. The absorbance was measured at 540 nm using the automated MRX microplate reader (Dynatech laboratories UK). Absorbance of control wells was taken as 100%, and the results were expressed as a percent of control.
Cell cycle analysis
Cell cycle distribution in Caco-2 cells treated with berberine chloride at concentrtion 5 M for 72 h was analyzed by propidium iodide (PI) DNA staining using a Cycle TESTTM PLUS DNA Reagent Kit (Becton Dickinson, San Jose, CA, USA). After treatment, cells were harvested and washed twice in PBS. Then, they were processed and stained according to the manufacturer’s instructions. After staining, samples were immediately acquired on a FACS Vantage SE flow cytometer using CellQuest software (Becton Dickinson, San Jose, CA, USA). A minimum of 1 × 104 events was analyzed per analysis. PI fluorescence was detected in the pulse-processed FL3 channel (630/22 nm band pass filter). Results were analyzed using ModFit LT 3.0 software. Percentages of cells corresponding to G0/G1, S and G2/M phases of the cell cycle were calculated. The cells with a DNA content lower than that of G1-phase cells (hypoploid population) were considered to be apoptotic (sub-G1). Performance of the instrument and sensitivity of measurement were checked prior to acquisition by using a DNA QC Particle Kit (Becton Dickinson, San Jose, CA, USA).
Annexin V/PI staining
The assay was performed as described previously [29]. Briefly, 5 × 105 Caco-2 cells after berberine chloride exposure (5µM for 24, 48 and 72 h) were washed twice in PBS and resuspended in 100 µl of binding buffer (Becton Dickinson, San Jose, CA, USA). The cells were subsequently stained with annexin V-FITC (An) and PI (Becton Dickinson, San Jose, CA, USA) according to the manufacturer’s instructions. After staining, the cells were resuspended in 400 µl of binding buffer and minimum 1 x 104 events was acquired immediately using the FACS Vantage SE flow cytometer (Becton Dickinson, San Jose, CA, USA). Annexin V-FITC and PI fluorescences were detected in FL1 (530/30 nm band pass filter) and FL3 (630/22 nm band pass filter) channels. Samples were acquired and analyzed using the CellQuest software (Becton Dickinson, San Jose, CA, USA). The results are divided into viable, An–/ PI– cells; early apoptotic cells with preserved plasma membrane integrity (An+/PI–), late apoptotic cells which have lost their plasma membrane integrity and have become An+/PI+).
Statistical analysis
Results are expressed as mean ± standard deviation (SD). Statistical analysis of the data was performed with one-way ANOVA followed by Bonferroni multiple comparisons test. Values of p<0.05 were considered to be statistically significant.
Results
Assessment of cytotoxicity and potentiation of cytotoxic drugs activity by MTT assay
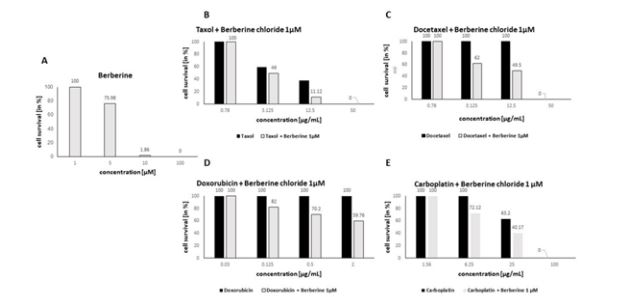
Our data has shown that berberine chloride possesses antiproliferative activity. The highest effectivity was observed in inhibition of proliferation Caco-2 cells at concentration 100 μM and 10 μM with efficiency almost 100 % and with IC50 = 6.7526 μM. Monitoring of the effect of berberine chloride at ineffective concentration 1 μM in combination with selected cytostatics, we observed that in combination with taxol, the IC50 value decreased to 3.1 μg/mL compared to the original 7.13 μg/mL, which was achieved with taxol alone, combination with docetaxel decreased IC50 to 12.13 μg/mL from 31.13 μg/mL. Doxorubicin showed no inhibitory effect on Caco-2 cell growth even at the highest concentration tested. Cotreatment with berberine chloride (1 μM) resulted in a 40% reduction in cell survival at the highest tested doxorubicin concentration 2 μg/mL and at a concentration of 0.5 μg/mL by 30%. Treatment of Caco-2 cells with cisplatin revealed IC50 = 40.67 μg/mL, which was reduced to 19.23 μg/mL with berberine chloride (1 μM).
Figure 1: The effects of tested berberine chloride in Caco-2 cell line alone (A) at concentrations 100 – 0.1 μM and in combination with taxol (B), docetaxel (C), doxorubicin (D) and carboplatin (E) at concentration 1μM after 72 h incubation.
Cell cycle analysis
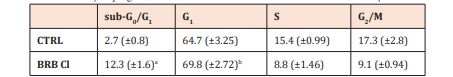
Cell cycle analysis of Caco-2 cells exposed to berberine chloride at a concentration 5 μM for 72 h exhibited an increase in sub-G0/G1 fraction considered as apoptotic and very mild increase in G0/G1 fraction followed by decrease in S and G2/M fraction. Quantification of the percentage of cells in the different cell cycle phases is shown in Table 1.
Table 1: Effect of berberine chloride on the cell cycle progression of Caco-2 cells 72 h incubation at concentration 5 μM.
The results are presented from three independent experiments as mean ± SD; (Significance vs. untreated control: ap<0.05, bp<0.01, and cp<0.001).
Annexin V/PI staining
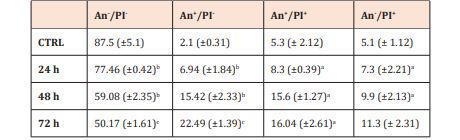
To confirm the proapoptotic effect of berberine chloride at a concentration 5 μM on Caco-2 cells we performed Annexin V staining to detect phosphatidylserine (PS) externalization and PI staining do detect increase in nuclear membrane permeability. Analysis of Caco-2 cells treated with 5 μM of berberine chloride for 24, 48 and 72 h showed a significant increase in the An+ / PI+ cells considered late apoptotic and An + / PI- cells considered early apoptotic. After 24 h of incubation, nearly 7 % of the cells were observed in the early phase of apoptosis (An + / PI-) and 8 % in the late stage of apoptosis (An+ / PI+). With prolonged time of incubation, the percentage of cells increased and after 72 h, almost 23 % of Caco-2 cells was found in the early phase and 16 % in the late phase of apoptosis (Table 2).
Table 2: Induction of apoptosis in Caco-2 cells after treatment with berberine chloride (5 μM) using Annexin V-FITC/PI staining.
The results are presented from three independent experiments as mean±SD. Living cells are presented as An-/PI- events, cells in the early apoptotic stage are presented as An+/PI-, late apoptotic cells as An+/PI+ and death cells as An-/PI+ (significance to untreated control: ap<0.05, p<0.01, cp<0.001).
Discussion
Colorectal cancer, despite advances in treatment and diagnosis, is one of the most common causes of death from cancer in industrialized countries and the success of its treatment in terms of patient survival is below 40 % [30]. Patients must deal with treatment-related symptoms [31] as well as with adverse effects associated with chemotherapy because antitumor agents also affects other organs, therefore, new strategies are needed to reduce the risk of complications caused by antineoplastic treatment, while maintaining or increasing their therapeutic effects. Zhao et al [32] reported that berberine pretreatment in in vivo experiments on mice showed hepatoprotective effect on doxorubicin-induced liver injury [32,33]. Pretreatment with berberine also ameliorated the doxorubicininduced kidney injury in rats, which could represent the potential for a new strategy to prevent doxorubicin-associated hepatocellular toxicity [33]. The doxorubicin-induced cardiotoxicity was effectively reduced in rats by berberine, which inhibited the metabolism of doxorubicin and reduced accumulation of doxorubicinol [34]. Another in vitro study provided by Hur et al. [35] documented significantly enhanced antitumor effect of gamma radiation in the hepatocellular carcinoma cells treated with the combination of berberine and irradiation as well as in the irradiated cells pretreated with berberine [35].
The effect of cisplatin or 5-fluorouracil effect in larynx squamous carcinoma cells was enhanced after 4h pre-treatment with berberine [36]. Cotreatment with berberine and cisplatin in ovarian cancer cells induced apoptosis and necroptosis leading to significantly increased anticancer effect [37]. Our data showed that combination of conventional cytostatics taxol, docetaxel, doxorubicin and carboplatin with berberine chloride at ineffective concentration increaced its antiproliferative activity and decreased their IC50 values. Several mechanisms contributing to the antitumor activity of berberine have been described including cell cycle arrest and induction of apoptosis. We noticed significant increase in sub-G0/G1 proapoptotic fraction and only very mild increase in G0/G1 phase in berberine treated Caco-2 cells. Liu et al. [38] observed G1 arrest – dependent on p53, and also G2/M arrest – regardless of the status of p53, in human osteosarcoma cells exposed to berberine, whereas apoptosis contributed to the antiproliferative effect of berberine to a lesser extent in this study [38]. In our experiments we noticed significantly increased number of Caco-2 cells in early and late apoptotic stage after treatment with berberine chloride.
Based on the mentioned results, we can assume that berberine has chemopreventive effects and the combination of berberine with cytostatics could be a promising alternative treatment for cancer.
Conclusion
Based on our in vitro data we may conclude that berberine chloride induces apoptosis in colorectal carcinoma cells and significantly increases antiproliferative activity of of conventional cytostatics taxol, docetaxel, doxorubicin and carboplatin. Despite the fact that we did not observe the biphasic effect of berberine in our experiments, it is still necessary to perform many studies dealing with the issue.
References
- http://globocan.iarc.fr. In
- Oberlies NH, Kroll D J (2004) Camptothecin and taxol: historic achievement in natural products research. J Nat Prod 67 (2): 129-135.
- Cragg G M, Newman D J, Snader K M (1997): Natural products in drug discovery and development. J Nat Prod 60 (1): 52-60.
- Potdar D, Hirwani R R, Dhulap S (2012) Phyto-chemical and pharmacological applications of Berberis Aristate. Fitoterapia 83(5): 817-830.
- Singh A, Duggal S, Kaur N, Singh J (2010) Berberine: Alkaloid with wide spectrum of pharmacological activities. J Nat Prod 3: 64-75.
- Habtemariam S (2013) The hidden treasure in Europes garden plants: Case examples Berberis darwinni and Bergenia cordifolia. Med Arom Plants 2: 4.
- Habtemariam S (2011) The therapeutic potential of Berberis darwinii stem-bark: Quantification of berberine and in vitro evidence for Alzheimers disease therapy. Nat Prod Commun 6(8): 1089-1090.
- Singh A, Bajpai V, Srivastava M, Arya K R, Kumar B (2015) Rapid screening and distribution of bioactive compounds in different parts of Berberis petiolaris using direct analysis in real time mass spectrometry. J Pharm Anal 5(5): 332-335.
- Suau R, Rico R, López-Romero J M, Nájera F, Cuevas A (1998) Isoquinoline alkaloids from Berberis Vulgaris subsp. Aus Phytochem 49(8): 2545-2549.
- Lv X, Li Y, Tang C, Zhang Y, Zhang J, et al. (2016) Integration of HPLC-based fingerprint and quantitative analyses for differentiating botanical species and geographical growing origins of Rhizoma coptidis. Pharm Biol 54(12): 3264-3271.
- Brown P N, Roman M C (2008) Determination of Hydrastine and Berberine in Goldenseal Raw Materials, Extracts, and Dietary Supplements by High-Performance Liquid Chromatography with UV: Collaborative Study. J AOAC Int 91(4): 694-701.
- Weber H A, Zart M K, Hodges A E, Molloy H M, O Brien B M, et al. (2003) Chemical comparison of goldenseal (Hydrastiscanadensis L.) root powder from three commercial suppliers. J Agric Food Chem 51(25): 7352-7358.
- Jamshaid F, Dai J, Yang LX (2020) New development of novel berberine derivatives against bacteria. Mini Rev Med Chem 20(8): 716-724.
- Habtemariam S (2016) Berberine and inflammatory bowel disease: A concise review. Pharmacol Res 113(Pt A): 592-599.
- Liang Y, Xu X, Yin M, Zhang Y, Huang L, et al. (2019) Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr J 66(1): 51-63.
- Tabeshpour J, Imenshahidi M, Hosseinzadeh H (2017) A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran J Basic Med Sci 2017, 20(5): 557-568.
- Shinjyo N, Parkinson J, Bell J, Katsuno T, Bligh A (2020) Berberine for prevention of dementia associated with diabetes and its comorbidities: A systematic review. J Integr Med 18(2): 125-151.
- Rabbani GH, Butler T, Knight J, Sanyal SC, Alam K (1987) Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholerae. J Infect Dis 155(5): 979-984.
- Iizuka N, Miyamoto K, Okita K, Tangoku A, Hayashi H, et al. (2000) Inhibitory effect of Coptidis Rhizoma and berberine on the proliferation of human esophageal cancer cell lines. Cancer Lett 148(1): 19-25.
- Jantova S, Cipak L, Cernakova M, Kostalova D (2003) Effect of berberine on proliferation, cell cycle and apoptosis in HeLa and L1210 cells. J Pharm Pharmacol 55(8): 1143-1149.
- Lin J P, Yang J S, Lee J H, Hsieh W T, Chung J G (2006) Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J Gastroenterol 12(1): 21-28.
- Mantena S K, Sharma S D, Katiyar S K (2006) Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther 5(2): 296-308.
- Mantena S K, Sharma S D, Katiyar S K (2006) Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis 27(10): 2018-2027.
- Piyanuch R, Sukhthankar M, Wandee G, Baek S J (2007) Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett 258(2): 230-240.
- Sanders M M, Liu A A, Li T K, Wu H Y, Desai S D, et al. (1998) Selective cytotoxicity of topoisomerase-directed protoberberines against glioblastoma cells. Biochem Pharmacol 56(9): 1157-1166.
- Liu Z, Liu Q, Xu B, Wu J, Guo CH, et al. (2009) Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat Res 662(1-2): 75-83.
- Bao J, Huang B, Zou L, Chen S, Zhang C, et al. (2015) Hormetic effect of berberine attenuates the anticancer activity of chemotherapeutic agents. PLOS ONE 10(9): e0139298.
- Mosmann T (1983), Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1-2): 55-63.
- Kravtsov V D, Greer J P, Whitlock J A, Koury M J (1998) Use of the microculture kinetic assay of apoptosis to determine chemosensitivities of leukemias. Blood 92(3): 968-980.
- Kuipers E J, Grady W M, Lieberman D, Seufferlein T, Sung J J, et al. (2015) Colorectal cancer. Nat Rev Dis. Primers 1: 15065.
- Coolbrandt A, Dierckx DE Casterlé B, Wildiers H, Aertgeerts B, et al. (2016) Dealing with chemotherapy-related symptoms at home: a qualitative study in adult patients with cancer. Eur J Cancer Care 25(1): 79-92.
- Zhao X, Zhang J, Tong N, Chen Y, Luo Y (2012) Protective effects of berberine on doxorubicin-induced hepatotoxicity in mice. Biol Pharm Bull 35(5): 796-800.
- Chen X, Zhang Y, Zhu Z, Liu H, Guo H, et al. (2015) Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Molecular Medicine Reports 13(5): 3953-3960.
- Hao G, Yu Y, Gu B, Xing Y, Xue M (2015) Protective effects of berberine against doxorubicin-induced cardiotoxicity in rats by inhibiting metabolism of doxorubicin. Xenobiotica 45(11): 1024-1029.
- Hur J M, Hyun M S, Lim S Y, Lee W Y, Kim D (2009) The combination of berberine and irradiation enhances anti-cancer effects via activation of p38 MAPK pathway and ROS generation in human hepatoma cells. J Cell Biochem 107(5): 955-964.
- Palmieri A, Iapichino A, Cura F, Scapoli L, Carinci F, et al. (2018) Pre-treatment with berberine enhances effect of 5-fluorouracil and cisplatin in HEP2 laryngeal cancer cell line. J Biol Regul Homeost Agents 32 (2 Suppl. 1): 167-177.
- Liu L, Fan J, Ai G, Liu J, Luo N, et al. (2019) Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol Res 52(1): 37.
- Liu Z, Liu Q, Xu B, Wu J, Guo C, et al. (2009) Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat Res 662(1-2): 75-83.