Kinetics of Inflammatory and Pain Biomarkers during Self-Management Program Treatment of Temporomandibular Disorders – Myofascial Pain Syndrome: A Prospective Observational Study
Flávia Fonseca Carvalho Soares1, Elizete Maria Rita Pereira2, Elisa Carvalho de Siqueira3, Fabrício Tinôco Alvim de Souza1, Letícia Martins Guimarães1, Renan Pedra de Souza4, José Mario Neto Soares5, Marcus Vinícius Gomez2, Ricardo Santiago Gomez1 and Carolina Cavalieri Gomes3
1Department of Oral Surgery and Pathology, Universidade Federal de Minas Gerais, Brazil
2Institute of Education and Research Santa Casa Belo Horizonte, Laboratory of Toxins, Brazil
3Department of Pathology, Universidade Federal de Minas Gerais, Brazil
4Department of General Biology, Universidade Federal de Minas Gerais, Brazil
5Department of Restorative Dentistry, Universidade Federal de Minas Gerais, Brazil
*Corresponding author: Flávia Fonseca Carvalho Soares, Department of Oral Surgery and Pathology, School of Dentistry, Universidade Federal de Minas Gerais, Av. Antonio Carlos, 6627, Belo Horizonte, MG, CEP 31270 901, Brazil
Article History
Received: February 08, 2021 Accepted: April 02, 2021 Published: April 05, 2021
Citation: Soares FFC, Pereira EMR, Siqueira EC, et al. Kinetics of Inflammatory and Pain Biomarkers during Self-Management Program Treatment of Temporo mandibular Disorders – Myofascial Pain Syndrome: A Prospective Observational Study. Int. J. Orl. Health. 2021;1(1):09‒14. DOI: 10.51626/ijoh.2021.01.00003
Abstract
Objectives: Investigate the salivary flow rate together with salivary levels of IL-6, IL-1β, substance P, glutamate and reactive oxygen species (ROS) in patients with temporomandibular disorders (myofascial pain) before and during self-management program. Moreover, evaluate the association of pain intensity with the biomarkers concentration.
Materials and method: Saliva of nineteen patients diagnosed with TMD-MD was collected before self-management program and during the treatment (4 and 8 weeks).
Results: No significant alteration of the expression levels of the biomarkers evaluated and the salivary flow rate throughout time was found. No correlation was found between pain intensity and the molecular parameters evaluated at any of the three moments.
Conclusion: the biomarkers evaluated and salivary flow rates did not change during eight weeks of self-management program intervention in the sample evaluated.
Keywords: Pain Biomarkers; Myofascial pain; Temporomandibular disorders; Salivary flow; Self-management program treatment
Abbreviations
TMD: Temporomandibular Disorders; ROS: Reactive Oxygen Species; TENS: Transcutaneous Electrical Stimulation; rTMS: Repetitive Transcranial Magnetic Stimulation; DIMST: Deep Intramuscular Stimulation Therapy
Introduction
Temporomandibular disorders (TMD) are defined as sign and symptoms collection that involve masticatory muscles, temporomandibular joint (TMJ) or both. They can affect 25 to 40% in general population, and the most common type of presentation is the TMD of muscular origin, defined as TMD muscle disorders (TMD-MD) [1]. TMD-MD includes mostly TMD myalgia and myofascial pain. It can lead to pain, decreased muscle strength, limited range of motion and, in some cases, decreased salivary flow rate [2,3]. This disorder interferes with many daily activities and the functional capacity of those affected. Thus, TMD-MD has a significant impact on the quality of life of those who suffer from its symptoms [4]. Although this condition is one of most common pain syndromes, it is yet poorly understood due to its multifactorial aetiology [5]. Biological, social and psychological factors are involved in the complex pathogenesis of TMD-MD.
Previous studies suggest that some biomarkers such as IL-6, IL-1β, glutamate, substance P and reactive oxygen species (ROS) participate in chronic pain mechanisms [6], but only a few studies investigated those in TMD-MD. Those biomarkers stimulate the opening of ion channels in nociceptors, firing pain threshold, causing hyperalgesia and pain, as the noxious stimulus continues to excite the nociceptor, the action potential is maintained by feedback from inflammatory and pain mediators that are released as a function of the nociceptor stimulation, chronifying the pain [7-10].
Among the proposed treatments to TMD-MD, there are pharmacotherapy, dry needling, transcutaneous electrical stimulation (TENS) in addition to others. In a recent study, Medeiros et al. [9] described the effects of two different treatments, repetitive transcranial magnetic stimulation (rTMS) and deep intramuscular stimulation therapy (DIMST), on the expression of some biochemical parameters [9]. They assessed TNF-alfa, IL-6, IL-10 and ROS in blood samples at baseline, on the fifth treatment day (after receiving the intervention), and on the tenth day (immediately before receiving the intervention). The interventions did not change the biochemical parameters blood levels [9].
Physiotherapy combined with cognitive/behavioural therapy, also know as self-management program [11], is a treatment modality for TMD-MD, which presents advantages over the others, including that it is simple to execute, reversible and non-invasive [12]. This modality of treatment aims to lead to pain control, reduce muscular tone and improve kinetic and functional parameters of the temporomandibular joint. However, there is no data on the effects of such therapy on the salivary expression of pain mediators such as IL-6, IL-1β, glutamate, substance P and ROS. In addition, it is not known if this treatment modality impacts in salivary flow, as reported previously in other TMD treatment types [2,3]. This information could help to clarify the effects of self-management program in this clinical parameter. Therefore, the aim of the present study was to investigate salivary flow rate, as well as the levels of pain biomarkers, in the saliva of patients diagnosed with TMD-MD before and during self-management program treatment. Also, we tested the association of pain intensity with the concentration of the biomarkers. We hypothesized that self-management program treatment modulated pain, leading to pain biomarkers’ reduction, as well as to salivary flow increase. Although the determination of the levels of these substances in the saliva cannot directly serve as diagnostic biomarkers for the spontaneous pain, the understanding of their kinetics during self-management program treatment can contribute to the understanding of the biological basis this condition treatment.
Materials and Method
This study was carried out according to STROBE (Strengthening the Reporting of Observational studies in Epidemiology) (accessed at https://www.strobe-statement.org/index.php?id=strobe-home).
Patient recruitment and study design
Individuals were recruited at the School of Dentistry of Universidade Federal de Minas Gerais. A convenience sample of patients diagnosed with muscle disorders, myalgia or myofascial pain, according to the criteria defined by the Research Diagnostic Criteria for Temporomandibular Disorders (RDC-TMD) [13]. Were eligible to participate in the study. The group of muscle disorders was called Temporomandibular Disorders – Muscle Disorder (TMD-MD). The eligibility criteria was the diagnosis of TMD-MD, the protocol treatment and study participation acceptance. Those who were suffering from other odontogenic pain such as pulpitis, and/or neuropathic pain; generalized/regional pain such as fibromyalgia and/or whiplash-associated disorders; diagnosed with periodontitis and/or systemic inflammatory disease; were under analgesic or anti-inflammatory use during treatment, were under orthodontic treatment and/or were under another kind of treatment for TMD were excluded. The sources of medical information was according previous medical exams, when there was some doubt about a medical diagnostic, the individual was excluded from the study.
The eligible patients answered the validated version for the Portuguese language of the questionnaire RDC-TMD and were invited to take part in the study, composing a convenience sample. The study was conducted after obtaining approval from the Research Etic Committee of Universidade Federal de Minas Gerais (protocol CAEE: 50915415.1.0000.5149), and the patients signed informed consent forms after agreeing to participate.
The non-stimulated saliva collection method was chosen to avoid the induction of increased muscular pain in the individuals and also to avoid interferences in the protein analysis in the total saliva. Non-stimulated saliva of each patient was collected at three different moments: first, day 0 (before starting treatment, at the day of diagnosis), second time after four weeks of treatment, and third time, eight weeks after starting treatment. The treatment was conducted according the department protocol. The participants who abandoned the study and, therefore, did not have their saliva collected at three moments were excluded from the study. The patient’s chronic pain grade classification was assessed according to the graded chronic pain scale (GCPS) and pain intensity was scored according to a pain scale in which zero means “no pain” and ten “the worst possible pain”, both scales available at the RDC-TMD questionnaire. Details of saliva collection and parameters evaluated are described below.
Self-management program treatment
The participants were instructed, to follow the treatment and received written instructions. The treatment consists in the change of habit of clenching teeth during the waking period by focusing on the habit of maintaining the jaw in habitual position: tongue slightly located in the anterior palate with the teeth in disocclusion, keeping the functional space free, and stretching of the masticatory and neck muscles for about 10s, five times each session, three sessions a day [11,12,14-17].
Saliva collection
Human saliva mirrors the body’s health, so, it is been used as a diagnostic tool and in researches on developing technologies for the detection of biomolecules [18,19]. The following collection method has already been standardized in other works [20].
Patients were instructed not to eat or drink liquids for 60 minutes before saliva collection. After this, at the clinics, the patient’s mouth was rinsed with water, and he/she remained at rest state, without swallowing, chewing or speaking, sitting with his torso facing forward for two minutes. All saliva accumulated during this period was discarded by swallowing, and from that moment the patient was instructed to spit in a sterile 50 ml Falcon tube for 10 minutes. All saliva collections were performed on ice and in the morning period, between 9:00 and 11:00 am.
The saliva was centrifuged at 1129G for 15 minutes at 4ºC, and then the supernatant was measured by micropipettes and diluted 1:1 in PBS solution (0.4mM NaCl and 10mM NaPO4) containing protease inhibitors. It was kept frozen at -80°C for up to six months prior to analysis.
Analysis of pain biomarkers
Total protein concentration of each sample was measured by using standard Bradford method, and then the total values obtained were used to correct the values of each sample in ELISA’s and glutamate dosing assay. The concentration of IL-6, IL-1β and substance P were measured by using Human DuoSet ELISA Kit (IL-6’s Catalog Number: DY206-05; IL-1β’s Catalog Number: DY201-05, R&D Systems Inc, USA) and Substance P ELISA Kit (Kit number 583751, Cayman Chemical, USA), respectively, in an EpochTM Microplate Spectrophotometer (BioTek, Winooski, USA). Glutamate concentration was measured by the enzymatic method, following the increase in the fluorescence due to the production of NADPH in the presence of glutamate dehydrogenase (GDH) and NADP+ [21] using spectrophotometry (Shimadzu, model RF-5301). ROS was dosed by the experimental method of DCF-DA’s deacetylation (6 HALLIWELL, GUTTERIDGE, 2007) using a microplate reader (VICTOR 4X, Perkin Elmer, Waltham, USA). IL-6, IL-1β and ROS concentrations were performed in replicates, and interleukins and ROS of all samples for each patient were evaluated on a same experiment, to avoid the “batch effect” [22].
Statistical analysis
Ordinal (pain intensity scale) and continuous (biomarkers’ concentration and salivary flow rate) outcomes were represented using median and interquartile range. Mann-Whitney test compared medians of two independent groups. Friedman’s test was used to compare medians of longitudinally measured outcomes. Continuous variable associations were assessed using Spearman’s correlation coefficient. Analyses were carried out using R version 3.3.2 with nominal significance level of 0.05. Bonferroni corrected significance level was set at 0.001.
Results
Patients characterization
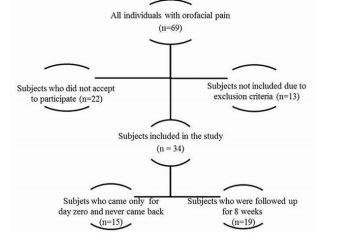
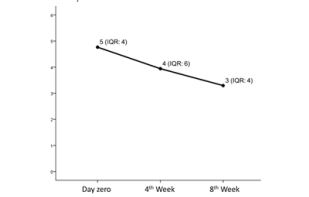
Sixty-nine individuals met the inclusion criteria, and thirteen met exclusion criteria of the study, but only thirty-four accepted to take part in the study, of which, fifteen came only at day zero, and 19 came on day zero, 4th and 8th weeks, according to a flux diagram shown in Figure 1. Twenty-two individuals did not accept to participate in the study, being the main reason for that time incompatibility with the appointments in the morning period. Besides that, we had 15 drop-outs probably in function of pain symptoms improvement, once the subjects claimed that was not needed to come back for follow-ups when they were contacted by phone. Pain intensity reported at the day of TMD-MD diagnosis by the group that came only once (at day zero) was not statistically different from the 19 patients included in the study (p>0.05). Figure 2 shows the median of pain intensity score during the proposed treatment. Although there was a decrease in pain intensity the difference did not reach statistical significance (p>0.05).
Figure 1: Flux diagram of individuals eligible for the study. After applying exclusion and inclusion criteria and participants’ loss, 19 individuals were included in the study.
Figure 2: Median of pain intensity score (median and IQR value) before treatment (measured at day zero) and self-management treatment (4th week and 8th week). Axis X represents pain intensity score graduated from zero to
10. There was no difference in pain intensity score before and during treatment (p>0.05, Friedman Test).
The sample group consisted of 19 individuals diagnosed with TMD-MD who were followed-up for eight weeks, presenting a median age of 34 year-old, ranging from 21 to 60 year-old. The sample showed a predominance of female individuals (89.5%). Regarding chronic pain grade classification, 58% of the sample showed high disability and moderate limitation (III grade), 31.5% presented low disability and high pain intensity (II grade) and 10.5% low disability and low pain intensity (grade I). Pain chronicity time ranged from one year to 25 years.
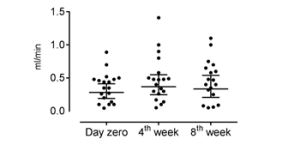
Figure 3: : Salivary flow rate before treatment (measured at day zero) and during self-management program treatment (4th week and 8th week). Axis X represents the three moments of collection of saliva and axis Y represents the salivary flow rate in milliliter per minute. There was no difference in the salivary flow rates
before and during treatment (p>0.05, Friedman Test).
Molecular parameters vs clinical parameters
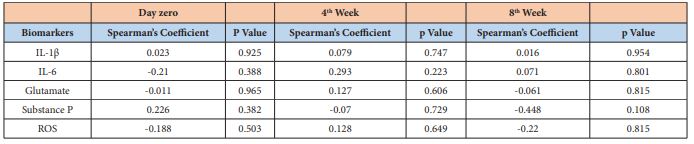
An increase in salivary flow was observed during the proposed treatment (Figure 3), but it did not reach no statistical significance (p>0.05). No correlation was found between pain intensity and the molecular parameters evaluated at any of the three moments (day 0, week 4 and week 8) (p>0.05; 95% of confidence intervals) as shown in Table 1.
Table 1: : Correlation between pain intensity reported by the individual and the biomarker’s concentration in each stipulated moment. It was used 95% of confidence intervals.
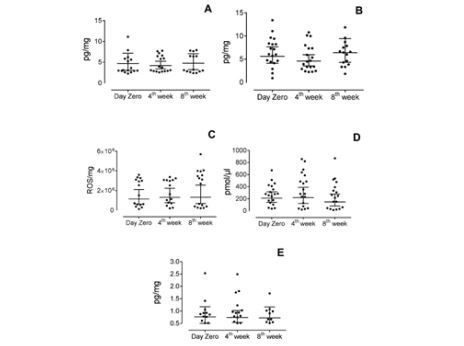
The kinetics of the five biomarkers according to time is shown in Figure 4. There was a variation of the median salivary concentration of each biomarker among the three saliva collections, which follows 3.12 pg/mg (IQR 3.09) day zero, 3.76 pg/mg (IQR 3.66) 4th week and 3.21 pg/mg (IQR 4.86) 8th week for substance P; 6.06 pg/mg (IQR 3.97) day zero, 4.5 pg/mg (IQR 4.45) 4th week and 6.52 pg/mg (IQR 5.54) 8th week for IL1-β; 1506047 ROS/pg (IQR 2578246) day zero, 1634916 ROS/pg (IQR 2375204.33) 4th week and 2916197 ROS/pg (IQR 3476536.25) 8th week for ROS; 278.32 pmol/µl (IQR 275.06) day zero, 280.85 pmol/µl (IQR 510.99) 4th week and 231.72 pmol/µl (IQR 370.42) 8th week for glutamate and 0.76 pg/mg (IRQ 0.48) day zero, 0.78 pg/mg (IQR 0.53) 4th week and 0.61 pg/mg (IQR 0.56) 8th week for IL-6. It was used 95% of confidence intervals. However, the statistical analyses did not reveal significance for these differences at the three moments for any of the five biomarkers (p>0.05). None of the presented results remained significant after multiple testing corrections.
Figure 4: : Kinetics of each evaluated biomarker during the study. Levels of Substance P (A), IL-1β (B), ROS (C), Glutamate (D) and IL-6 (E) in saliva of individuals with TMD-MD before (day zero) and during self-management program treatment (measured after 4 weeks and 8 weeks). Axis X represents the three moments of the collection of saliva and axis Y represents the concentration of the biomarkers in picogram per milligram, except for (C), in which axis Y represents arbitrary units of fluorescence per milligram and in (D) picomol per microliter. No statistically significant difference was found for the differences in each individual marker before and during treatment *p>0.05 (Friedman Test).
Discussion
Pain biomarkers have scarcely been investigated in TMD-MD, especially myofascial pain syndrome (MPS) [7-10]. Case-control studies have shown high concentrations of nociceptive mediators in blood and decreassed salivary flow rate in individuals diagnosed with TMD when compared to healthy patients, but those parameters have not been prospectively evaluated [7-10]. The study of salivary pain biomarkers can not only clarify pain mechanisms, but also help in the identification of potential therapeutic targets [23]. To date the effects of self-management program treatment on biochemical parameters and salivary flow in TMD-MD patients is unknown. We assessed the salivary levels of markers of chronic pain in TMD-MD individuals, as well as the kinetics of those mediators during self-management program therapy. It is important to notice that human saliva is a blood reflection and it is been used in bioinformatics, metabolomics, genomics and proteomics researches cause has the ability to mirror both oral and systemic conditions [18]. When compared to blood, saliva contains smaller quantity of proteins, but since our study used a standard collection of saliva and always in the same individuals, the possible differences that could be found would be proportional to that protein concentration present in the saliva. It is worth mentioning that saliva has been previously proven to be a valuable source of nociceptive mediators to be used in study of chronic pain disorders, including TMD [18-24-27].
We assessed the concentrations of five biomarkers in total saliva, once before self-management program treatment and twice during it. Each of these markers is important in different pain mechanisms. While ROS is an indicator of oxidative stress, IL-6 and IL-1β participate in inflammatory pain [28], substance P is important in neurogenic inflammation, and glutamate is a neurotransmitter involved in peripheral and central nociception [29]. We hypothesised that the salivary concentration of IL-6, IL1β, substance P, ROS and glutamate would decay throughout treatment time, whereas the salivary flow would rise. However, our data did not confirm our hypothesis and we did not find any significant alteration among the five biomarkers throughout time (considering each subject individually). Morever, the salivary flow rate did not present any significantly change, as previously reported in two different occlusal appliance treatment of TMD-MD [3].
Medeiros et al. [9] evaluated the effect of rTMS and DIMST’s treatment on biochemical parameters in blood samples in individuals diagnosed with MPS and no significant alteration was also observed [9]. Moreover, we could not prove any association between the molecular parameters and pain intensity at any given moment. We have to consider that this is a pilot study, with a small sample size. Therefore, it is possible that lack of observed associations may be due low statistical power and not necessarily due to their true absence.
Molecular studies in TMD field for the most part concentrate in potential biomarkers present in synovial fluid of temporomandibular joints [30-32]. These studies concluded that clinical symptomatology, like pain intensity, could not be correlated with the biomarkers concentration [33,34]. In the context of muscular pain TMD, our data corroborates with those results.
We have to be cautious not to further over interpret our results, as our sample size is small. We speculate that the group of individuals who continued the treatment until the 8th week probably included some individuals refractory to the treatment. We acknowledge that the adherence to home-exercise treatment is still a challenge. Future studies should include other methods, beyond the physicians recommendations at the appointment, to assure the adherence to the home-exercise treatment like text-messages and an exercise diary. One has to consider that the moments defined for the second and third saliva collection were arbitrarily set as the 4th and 8th treatment week, as there is not currently established protocol in the literature. Despite these limitations, the exploratory nature of this study provides preliminary findings for future research.
Conclusion
In conclusion, our results suggest that IL-6, IL-1β, ROS, glutamate, substance P salivary levels and salivary flow rates do not change during eight weeks of cognitive/physiotherapeutic intervention. We could not prove an association between pain intensity and salivary concentration of these biomarkers. These preliminary results may guide new molecular works in temporomandibular disorders field, including a larger cohort of samples, stratification into subgroups and differentiated follow-up times.
Funding
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. RS Gomez, CC Gomes and FFC Soares are research fellows at CNPq.
References
- Wright EF (2000) Referred Craniofacial Pain Patterns in Patients with Temporomandibular Disorder. J Am Dent Assoc 131(9): 1307-1315.
- Le Bell Y, Söderling E, Kirveskari P, Alanen P (1985) Flow rate, pH and buffer capacity of whole saliva before and after treatment of TMJ dysfunction. Proc Finn Dent Soc 81(4): 226-229.
- Doepel M, Söderling E, Ekberg EL, Nilner M, Le Bell Y (2009) Salivary cortisol and IgA levels in patients with myofascial pain treated with occlusal appliances in the short term. J Oral Rehabil 36(3): 210-216.
- Gerwin RD (2001) Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep 5(5): 412-420.
- Rodriguez-Blanco C, Cocera-Morata FM, Heredia-Rizo AM, Ricard F, Almazán-Campos G, Oliva-Pascual-Vaca Á (2015) Immediate Effects of Combining Local Techniques in the Craniomandibular Area and Hamstring Muscle Stretching in Subjects with Temporomandibular Disorders: A Randomized Controlled Study. J Altern Complement Med 21(8): 451-459.
- Halliwell, B, Gutteridge JM (2007) Free Radicals in Biology and Medicine. (4th edn.), London, Oxford University Press.
- Basi DL, Velly AM, Schiffman EL, Lenton PA, Besspiata DA, et al. (2012) Human temporomandibular joint and myofascial pain biochemical profiles: A case-control study. J Oral Rehabil 39(5): 326-337.
- Grosman-Rimon L, Parkinson W, Upadhye S, Clarke H, Katz J, et al. (2016) Circulating biomarkers in acute myofascial pain. Medicine (Baltimore) 95(37): e4650.
- Medeiros LF, Caumo W, Dussán-Sarria J, Deitos A, Brietzke A, et al. (2016) Effect of Deep Intramuscular Stimulation and Transcranial Magnetic Stimulation on Neurophysiological Biomarkers in Chronic Myofascial Pain Syndrome. Pain Med 17(1): 122-135.
- Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, et al. (2008) Biochemicals Associated with Pain and Inflammation are Elevated in Sites Near to and Remote from Active Myofascial Trigger Points. Arch Phys Med Rehabil 89(1): 16-23.
- Durham J, Al-Baghdadi M, Baad-Hansen L, Breckons M, Goulet JP, et al. (2016) Self-management programmes in temporomandibular disorders: results from an international Delphi process. J Oral Rehabil 43(12): 929-936.
- Michelotti A, De Wijer A, Steenks M, Farella M (2005) Home-exercise regimes for the management of non-specific temporomandibular disorders. J Oral Rehabil 32(11): 779-785.
- Pereira Júnior FJ, Favilla EE, Dworkin SF (2004) Critérios de diagnóstico para pesquisa das disfunções temporomandibulares (RDC/TMD). Tradução oficial para a língua portuguesa. Bras Clin Odontol Integr 8: 384-495.
- Shimada A, Ishigaki S, Matsuka Y, Komiyama O, Torisu T, et al. (2019) Effects of exercise therapy on painful temporomandibular disorders. J Oral Rehabilitation 46(5): 475-481.
- Mulet M, Decker KL, Look JO, Lenton PA, Schiffman EL (2007) A randomized clinical trial assessing the efficacy of adding 6 x 6 exercises to self-care for the treatment of masticatory myofascial pain. J Orofac Pain 21(4): 318-328.
- Lindfors E, Arima T, Baad-Hansen L, Bakke M, De Laat A, et al. (2019) Jaw Exercises in the Treatment of Temporomandibular Disorders—An International Modified Delphi Study. J Oral Facial Pain Headache 39(4): 389-398.
- R F C P de Freitas, M Â F Ferreira, G A S Barbosa, P S Calderon (2013) Counselling and self-management therapies for temporomandibular disorders: A systematic review. J Oral Rehabil 40(11): 864-874.
- Liu J, Duan Y (2012) Saliva: a potential media for disease diagnostics and monitoring. Oral Oncol 48(7): 569-577.
- Topkas E, Keith P, Dimeski G, Cooper-White J, Punyadeera C (2012) Evaluation of saliva collection devices for the analysis of proteins. Clin Chim Acta 413(13-14): 1066-1077.
- De Souza FT, Amaral TM, Dos Santos TP, Abdo EN, Aguiar MC, et al. (2011) Burning Mouth Syndrome. A Therapeutic Approach Involving Mechanical Salivary Stimulation. Headache 52(6): 1026-1034.
- Nicholls DG, Sihra TS, Sanchez-Prieto J (1987) Calcium dependent and independent release of glutamate from synatosomes monitored by continuous fluorometry. J Neurochem 49(1): 50-57.
- Goh WWB, Wang W, Wong L (2017) Why Batch Effects Matter in Omics Data, and How to Avoid Them. Trends Biotechnol 35(6): 498-507.
- Rodríguez de Sotillo D, Velly AM, Hadley M, Fricton JR (2011) Evidence of oxidative stress in temporomandibular disorders: a pilot study. J Oral Rehabil 38(10): 722-728.
- Fischer HP, Eich W, Russell IJ (1998) A possible role for saliva as a diagnostic fluid in patients with chronic pain. Semin Arthritis Rheum 27(6): 348-359.
- Van Oosterhout WPJ, Schoonman GG, Garrelds IM, Danser AHJ, Chan KY, et al. (2015) A human capsaicin model to quantitatively assess salivary CGRP secretion. Cephalalgia 35(8): 675-682.
- Javaid MA, Ahmed AS, Durand R, Tran SD (2016) Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofacial Res 6(1): 67-76.
- Bazzichi L, Ciregia F, Giusti L, Baldini C, Giannaccini G, et al. (2009) Detection of potential markers of primary fibromyalgia syndrome in human saliva. Proteomics Clin Appl 3(11): 1296-1304.
- Khalil Z, Khodr B (2001) A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med 31(4): 430-439.
- Stanisz AM (2001) Neurogenic inflammation. Role of substance P. NeuroImmune Biol 1: 373-378.
- Gulen H, Ataoglu H, Haliloglu S, Isik K (2009) Proinflammatory cytokines in temporomandibular joint synovial fluid before and after arthrocentesis. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endo 107(5): 1-4.
- Herr MM, Fries KM, Upton LG, Edsberg LE (2011) Potential biomarkers of temporomandibular joint disorders. J Oral Maxillofac Surg 69(1): 41-47.
- Kellesarian SV, Al-Kheraif AA, Vohra F, Ghanem A, Malmstrom H, et al. (2016) Cytokine profile in the synovial fluid of patients with temporomandibular joint disorders: A systematic review. Cytokine 77: 98-106.
- Holmlund A, Ekblom A, Hansson P, Lind J, Lundeberg T, et al. (1991) Concentrations of neuropeptides substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid of the human temporomandibular joint. A correlation with symptoms, signs and arthroscopic findings. Int J Oral Maxillofac Surg 20(4): 228-231.
- Kaneyama K, Segami N, Sato J, Fujimura K, Nagao T, et al. (2007) Prognostic factors in arthrocentesis of the temporomandibular joint: comparison of bradykinin, leukotriene B4, prostaglandin E2, and substance P level in synovial fluid between successful and unsuccessful cases. J Oral Maxillofac Surg 65(2): 242-247.