Mass-Spectrometric Separation and Quantification of Permethrin and Cypermethrin Residues on Beans and Cucumber Plants
George F Antonious*
Division of Environmental Studies, Kentucky State University, USA
*Corresponding author: George F Antonious, Professor, College of Agriculture, Community and the Sciences, Division of Environmental Studies, 231A Cooperative Extension Building, USA
Article History
Received: February 04, 2021 Accepted: February 11, 2021 Published: February 16, 2021
Citation: Antonious GF. Mass-Spectrometric Separation and Quantification of Permethrin and Cypermethrin Residues on Beans and Cucumber Plants. Int J. Agri Res Env Sci. 2021;2(1):10‒14. DOI: 10.51626/ijares.2021.02.00006
Abstract
A simple, accurate, and cost-effective procedure for separation and quantification of two pyrethroid insecticides (permethrin and cypermethrin) residues on bean and cucumber plants grown under greenhouse conditions was achieved using a GC equipped with an electron capture detector (GC-ECD) and GC equipped with a mass selective detector (GC-MSD). Following spraying of a mixed formulation of the two insecticides, fruits and leaves were collected at different time intervals of 1 h to 25 d to determine insecticides dissipation constants (K values) and half-lives (T1/2 values). A simultaneous extraction procedure using hexane followed by cleaning up of plants crude extracts in an open glass chromatographic column packed with Florisil was developed. Residues of the two pyrethroids were confirmed using a GC-MSD in total ion mode. The GC-MSD revealed the presence of two permethrin isomers at retention times of 26 and 26.6 min that correspond to the cis- and trans-isomers, respectively. The GC-MSD also revealed the presence of four cypermethrin isomers at retention times of 30.3, 30.9, 31.3, and 31.5 min. The average initial deposits of permethrin were 2.7 and 0.2 on cucumber leaves and fruits surfaces, respectively. Whereas cypermethrin initial deposits were 5.1 and 2 µg g-1 on cucumber leaves and fruits, respectively indicating greater deposits on leaves than fruits. T1/2 values of permethrin and cypermethrin residues on beans pods (7.2 and 9.5 d, respectively) and cucumber fruits (13 and 3.3 d, respectively) indicated that a waiting period of 10 and 15 d are required for consumption of cucumber fruits and bean pods sprayed with cypermethrin at the recommended spraying dosage to drop the residues to the Maximum Residue Limits.
Keywords: Gas chromatography, Dissipation constants, Half-lives, Initial Residues, Maximum residue limits
Introduction
Cucumber plants are attacked by insect pests and diseases, the most important insects are aphids, Aphis gossypii Glover, Myzus persicae (Sulzer) (Hemiptera: Aphididae), the whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae), and the cucurbit borers Diaphania nitidalis (Cramer) and D. hyalinata (L.) (melonworm) (Lepidoptera: Crambidae) [1]. Bean plants are also subject to attach by spider mites, bean leaf beetles, aphids, whiteflies, leafhoppers, thrips, cutworms, earthworms, and stinkbugs [2] that require chemical control. Residues of pesticides on vegetables and fruits are a potential risk to consumers from short-term impacts (headaches and nausea) to chronic impacts, such as cancer, neurotoxicity, reproductive harm, and endocrine disruption [3]. Accordingly, fruits and vegetables have been given a lot of attention in monitoring programs since most of them are eaten uncooked.
Permethrin and cypermethrin are two insecticides commonly used in agricultural production systems. Permethrin is formulated and used as a blend of cis- and trans-isomers that have different chemical, physical, and toxicological properties [4]. Cypermethrin is also a synthetic pyrethroid (man-made insecticide) created to mimic the chemical properties of the naturally occurring insecticide pyrethrum. Synthetic pyrethroids, like cypermethrin kills bugs by affecting their nervous system. They are synthetic analogues of the natural pyrethrins derived from the flowers of pyrethrums (Chrysanthemum cinerariaefolium and C. coccineum). Pyrethrins represent 80% of the total market of botanical insecticides [5]. They are rapidly degraded by sunlight (photodegradation) and humidity (hydrolysis) under field conditions. There is a need to carry out selective monitoring of pesticide residues on vegetables grown under greenhouse conditions, where no direct contact with sunlight and rainfall events (that are usually provide adequate level of consumer protection). The mode of action of the majority of commercial pyrethroids is the disruption of voltage-sensitive sodium channels (VSSC) function in insect pests. All available evidence indicates that pyrethroids bind to the α subunit of the VSSC.
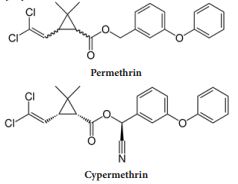
The major agricultural pyrethroids are esters of 3-phenoxybenzyl alcohol or α-cyano-3-phenoxybenzyl alcohol used as a single isomer (deltamethrin) or as a mixture of permethrin or cypermethrin isomers. Permethrin and cypermethrin are two insecticides commonly used in agricultural production in Kentucky and worldwide. They are very similar in chemical structure, but cypermethrin contains a cyanide (CN) group (Figure 1). Pyrethroids are sold and/or used commercially as a mixture containing a combination of two or more compounds (isomers) [6]. Permethrin [3-Phenoxybenzyl (1RS)-cis, trans- 3-(2, 2-dichlorovinyl) -2, 2-dimethylcyclopropanecarboxylate] and cypermethrin [cyano-(3-phenoxyphenyl) methyl] 3-(2, 2-dichloroethenyl)-2, 2-dimethylcyclopropane-1-carboxylate] are broad-spectrum pyrethroid insecticides most widely used in field crops and indoors for control of home pests. Permethrin is formulated as a blend of cis- and trans-isomers. It has a low potential for mammalian toxicology with an oral LD50 in rats ranging from 430 to 4000mgkg-1 as the cis: trans ratio changes from 40:60 to 20:80 [7]. The technical grade cypermethrin is the racemic mixture of 8 isomers (four cis and four trans isomers) Manna et al. [8]. The oral LD50 values in rats were 79mgkg-1 (5% in corn oil) and 40-80 mg kg-1 (10% in corn oil) [9]. The U.S. Department of Health and Human Service [10] and Manna et al. [8] reported that oral LD50 of α-cypermethrin, which is believed to be the most active isomer dissolved in dimethyl sulfoxide (DMSO) was 145 mg kg-1 in rats.
Isomers have different physical properties like boiling point, melting point, and solubility. Most commercial pyrethroids are not one single molecule; rather, they are several molecules with the same chemical formula that have their atoms joined together in the same sequence but have a different arrangement of the atoms in space. Such compounds (isomers) have different physical properties like boiling point, melting point, and solubility as well as different toxicities [10].The production of individual pyrethroids with slightly varying isomeric ratios can often be the reason for the differences in the reported toxicities of the same compound. Pyrethroids are grouped into two general classes, called Type I and Type II, based on a combination of toxicological and physical properties. Type I pyrethroids do not include a cyano-group at the α-carbon of the 3-phenoxybenzyl alcohol in their chemical structure, whereas Type II pyrethroids include a cyano- group (CN) as shown in Figure 1. Pyrethroids are very important insecticides because of their rapid paralysis of insect pests, have relatively low mammalian toxicity, and due to their rapid rate of degradation in the environment when exposed to sunlight. Thus, the production of individual pyrethroids with slightly varying isomeric ratios can often be the reason for the differences in the reported toxicities of the same compound [10].
Figure 1: Chemical structures of permethrin [3-Phenoxybenzyl (1RS)-cis, trans- 3-(2, 2-dichlorovinyl) -2, 2-dimethylcyclopropanecarboxylate] and cypermethrin [cyano-(3-phenoxyphenyl) methyl] 3-(2, 2-dichloroethenyl)-2, 2-dimethylcyclopropane-1-carboxylate].
Interconversion of the trans- and cis- isomers is a significant reaction on treated plants. Both isomers undergo ester cleavage and oxidation of the phenoxy group to produce the resulting acid and alcohol moieties. Accordingly, each active molecule may be sold as a mixture of different arrangements of two or more isomers, which represents a challenge for analytical chemists who should develop a chromatographic technique to separate each isomer before determining the persistence and T1/2 values of each compound. Pyrethroids are more stable, though this family of insecticides is generally degraded more rapidly in the environment, compared to other insecticides. All pyrethroids contain an acid moiety, a central ester bond, and an alcohol moiety. In general, type II compounds delay the inactivation of sodium channels substantially longer than do type I compounds [11]. However, the persistence of type I and type II compounds on treated plants under GH conditions (shaded areas) is unclear.
The objectives of this investigation were to:
a. separate permethrin and cypermethrin isomers using a single extraction and purification procedure,
b. determine the dissipation constants and T1/2 values of permethrin and cypermethrin residues on bean and cucumber leaves and fruits of plants grown under greenhouse conditions, and
c. determine fruit harvest time with respect to the maximum residue limits (MRLs) and greenhouse worker re-entry intervals.
Materials and Methods
Cucumber, Cucumis sativus var. Diva Hybrid (Family: Cucurbitaceae) and bean, Phaseolus vulgaris var. Lima (Family: Fabaceae) seedlings of five weeks old, that have two to four leaves were transplanted into silty loam soil in 32 diameter plastic pots under greenhouse conditions (20-23OC and 70% relative humidity). Cucumber and bean plants each in 48 pots were arranged in a randomized complete block design (CRBD) (twelve blocks and eight plants/block). Plants were watered as needed and fertilized every week with 200 ppm solution of Peters 20-20-20 NPK, a water-soluble fertilizer. All agricultural practices were applied according to the Vegetable Guide for Commercial Growers [2]. To support and encourage up and side plant shoot growing, vertical wires were put in place. Permethrin (36.8% AI) and cypermethrin (25.4% AI) formulations (obtained from Control Solutions, Inc., Pasadena, TX) were sprayed simultaneously at 3.6 and 5 mL/4 gallons of water, respectively when plants were 45 d old. Beans and cucumber leaves and fruits were collected at 1h,5,10,15,20, and 25d following praying for insecticides residue analysis.
Methods of Analysis
At each sampling time, 100g fruits or 50g leave samples (n=3 per treatment) were blended with hexane for one min for extraction of permethrin and cypermethrin residues deposited on the plant surfaces. After homogenization, the crude extract was decanted through a Buchner funnel that contained 55mm Whatman 934-AH glass microfiber filter discs (Fisher Scientific, Pittsburg, PA) and anhydrous Na2SO4. The crude extract was concentrated using a rotary vacuum evaporator (Buchi Rotovapor, Model 461, Flawil, Switzerland) at 35 OC, chased with nitrogen (N2) gas and stream evaporation and reconstituted in 10 mL of hexane. The hexane extracts were cleaned up using a 1.2 × 2mm i.d. open glass chromatographic column packed with 20g Florisil (60-100 mesh) and eluted with a 120mL of acetone: hexane (3:7 v/v) mixture. A portion of each purified extract was subsequently passed through a 0.4μm GD/X disposable syringe filter (Fisher Scientific, Pittsburg, PA). One μL (n=3) of this filtrate was injected into a gas chromatograph (GC) equipped with an electron-capture detector (ECD). GC separations were accomplished using a 25m × 0.20mm ID capillary column with 0.33μm film thickness (HP-1). Operating conditions were 230,250, and 280OC for injector, oven, and detector, respectively with a carrier gas (He) flow rate of 5.2 mLmin-1. Peak areas were determined using a Hewlett-Packard (HP) model 3396 series II integrator. Quantifications were based on average peak areas of 1μL injections obtained from external purified standard solutions of permethrin and cypermethrin obtained from Sigma- Aldrich Inc. (Saint Louis, MO, USA) and prepared in acetone-hexane mixture. Analytical grade insecticides were used for method calibration and insecticide residues were identified based on retention times (Rt values) of external standards.
Confirmation of Separated Peaks
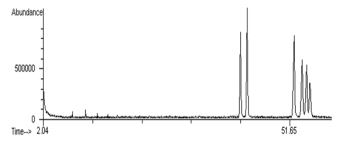
Peak identities were confirmed by consistent retention time and co-elution with standards under the conditions described above. A gas chromatograph HP model 5890A equipped with a mass selective detector (GC/MSD) operated in total ion monitoring with electron impact ionization (EI) mode and 70 eV (electron volt) also was used for identification and confirmation of individual peaks. The presence of permethrin and cypermethrin residues on plant tissues was confirmed using GC/MSD which showed molecular weight of 390 and spectral data with molecular ion peaks (M+) at m/z 183,168,163,153,127,91,77,51 and 27 along with characteristic fragment ion peaks that corresponds to permethrin fingerprints. The presence of cypermethrin residues on plant tissues was also confirmed using GC/MSD molecular weight of 415 and spectral data with molecular ion peaks (M+) at m/z m/z 209, 181,163,152,127,115, 91, 77, 65, 51, and 27 along with characteristic fragment ions that corresponds to cypermethrin. These detected mass spectral data are in agreements with those reported by the National Institute of Standards and Technology (NIST) for permethrin and cypermethrin [2].
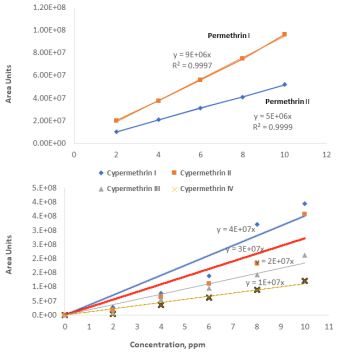
Residues of permethrin and cypermethrin detected using GC/ECD on cucumber and bean leaves and fruits were used to calculate regression slopes and T1/2 values using the equation T1/2 = ln 2/K, where K = -2.303 × slope of the line [12,13]. Under these GC/ECD conditions, retention times (Rt values) were 26.07 and 26.58min, for permethrin I (cis isomer) and permethrin II trans-isomer), respectively. Whereas cypermethrin Rt values were 30.3, 30.9, 31.3, and 31.5 min for its main four isomers, respectively. The chromatogram obtained using the GC/MSD (Figure 2) was used to identify and label isomers according to their elution time. Purified standards of the two pyrethroids (permethrin and cypermethrin) were injected into the GC-ECD and used as internal standards (Figure 3) for identification and quantification of each insecticide isomers and validation of the analytical method used. The standard materials were prepared at 0 to 10 µg mL-1 in n-hexane and stored at -18°C. The detection limits (minimum detectable concentration/ sample weight in grams) were higher (0.05 µg g-1) on the leaves compared to the fruits (0.01 µg g-1). The recovery values were 89 and 92 % for permethrin and cypermethrin on cucumber fruits and leaves, respectively. Whereas, the recovery values were 91 and 94% for permethrin and cypermethrin on bean fruits and leaves, respectively.
Figure 2: Gas chromatographic/mass spectrometric (GC/MS) chromatogram of permethrin and cypermethrin isomers detected in cucumber and bean leaves and fruits.
Results and Discussion
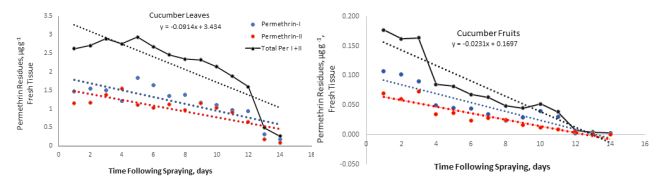
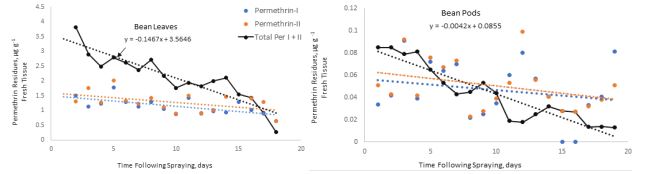
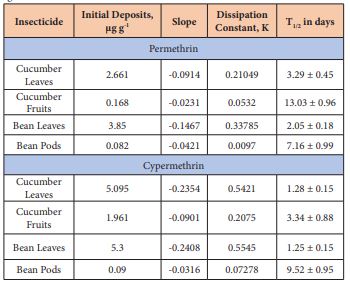
The present investigation provides a simple, accurate, and cost-effective procedure for separation and quantification of residues of two pyrethroid insecticides (permethrin and cypermethrin) on bean and cucumber plants using GC-ECD and GC-MSD methods. The residues of permethrin and cypermethrin decreased over time following spraying. The maximum concentration of permethrin residues on cucumber leaves and fruits was detectable one hour after spraying, and its concentration decreased with the increasing time following spraying. The initial residues of total cis- and trans-isomers of permethrin were 2.7 and 0.17 µg g-1 fresh weight on cucumber leaves and fruits, respectively (Table 1). These residues declined to 0.26 and 0.007 µg g-1 indicating dissipation constants of 0.21 and 0.05 on the leaves and fruits after 14 and 7 days, respectively (Figure 4). These dissipation patterns showed T1/2 values of 3.3 and 13 days on cucumber leaves and fruits, respectively revealing that the permethrin residue levels varied significantly between leaves and fruits tested and this could be due the high surface area of the leaves per unit weight of tissue compared to fruits as described by Antonious et al. [12].
Figure 3: Standard curves of permethrin I and permethrin II (upper graph) and cypermethrin I, II III, and IV used for quantification of residues on cucumber fruits and leaves.
Figure 4: Permethrin dissipation on cucumber leaves (upper graph) and cucumber fruits (lower graph) expressed as µg g-1 fresh tissue detected at different time intervals following a single spray of permethrin SFR formulation. Total permethrin indicates sum of the cis- (permethrin-I) and trans-(permethrin-II) isomers. Each value is an average of three replicate samples. Where no data is shown, indicates residues below the detectability limit.
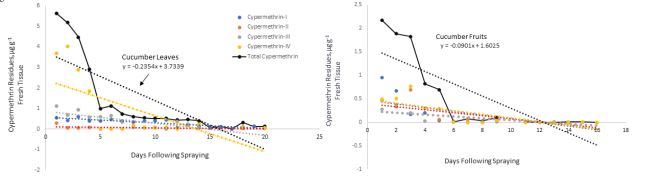
The initial deposits of total permethrin isomers were 3.9 and 0.08 µg g-1 fresh weight on bean leaves and fruits, respectively. These residues declined to 0.03 and 0.01 µg g-1 indicating dissipation constants of 0.34 and 0.01on bean leaves and fruits after 17 and 18 days, respectively (Figure 5). These dissipation patterns showed T1/2 values of 2.1 and 7.2 days on bean leaves and fruits, respectively. Regarding cypermethrin residues, Figure 6 revealed that the initial deposits of of total cypermethrin isomers on cucumber leaves and fruits were 5.1 and 2 µg g-1 fresh weight, respectively. The large surface area of the leaves per unit weight (gram) compared to fruits caused the low initial residues on the fruits.
Figure 5: Permethrin dissipation on bean leaves (upper graph) and bean pods (lower graph) expressed as µg g-1 fresh tissue detected at different time intervals following a single spray of permethrin formulation. Total permethrin indicates sum of the cis- (permethrin-I) and trans-(permethrin-II) isomers. Each value is an average of three replicate samples. Where no data is shown, indicates residues below the detectability limit.
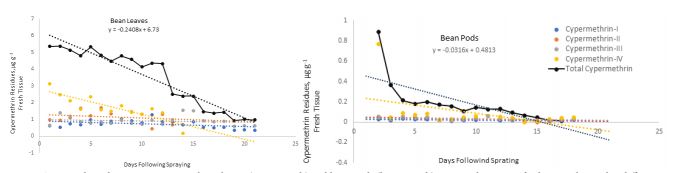
Residues of cypermethrin on cucumber was detected one hour after spraying, and its concentration decreased with the increasing the its dissipation. Permethrin and cypermethrin undergo ester cleavage to produce the pyrethroids acid and alcohol moieties. The initial residues of total cypermethrin isomers dropped from 5.8 and 2.2 into 0.1 and 0.0 µg g-1 fresh weight on cucumber leaves and fruits at 20- and 12-days following spraying, respectively (Figure 6). Cypermethrin residues declined from 5.3 and 0.86 to 0.9 and 0.00 µg g-1 on bean leaves and pods, respectively indicating dissipation constants of 0.55 and 0.07 after 21 and 17 days, respectively (Figure 7). This dissipation followed first-order kinetics, with T1/2 values of 1.3 and 9.5 days, respectively (Table 1) revealing that cypermethrin residue levels varied significantly between bean leaves and fruits and this also could be due the high surface area of the leaves per unit weight of tissue compared to the fruits.
Table 1: Initial deposits, dissipation constants, and half-lives (T1/2) of permethrin and cypermethrin on beans and cucumber grown under greenhouse conditions.
K = -2.303 × slope of the line; T1/2 = ln 2/K
According to the Joint FAO/WHO Joint Meeting on pesticides residues in food [14,15], the maximum permethrin residues limits (MRL) on cucumber fruits and shelled beans are 0.5 and 0.1µg g-1, respectively. Whereas the MRL cypermethrin residues on cucumber fruits and shelled beans are 0.2 and 0.05µg g-1, respectively. Pyrethroids and their isomers are non-systemic in plants (not translocated from the site of deposition nor can be translocated in the aerial parts of plants from soils). Accordingly, a waiting period of 6 days is suggested for consumption of cucumber fruits sprayed with permethrin at the recommended dosages to ensure that the residues are below the MRL (Figure 4). Whereas, no waiting period is required after spraying permethrin on bean pods (Figure 5). Regarding cypermethrin, a waiting period of 10 and 15 days are required for consumption of cucumber fruits and bean pods sprayed with cypermethrin at the recommended dosage to ensure that the residues are below the MRL (Figures 6 and 7, respectively).
Figure 6: Cypermethrin dissipation curves on cucumber leaves (upper graph) and cucumber fruits (lower graph) expressed as µg g-1 fresh tissue detected at different time intervals following a single spray of cypermethrin formulation. Total cypermethrin indicates sum of four isomers labeled according to their elution time. Each value is an average of three replicate samples. Where no data is shown, indicates residues below the detectability limit.
Figure 7: Cypermethrin dissipation curves on bean leaves (upper graph) and bean pods (lower graph) expressed as µg g-1 fresh tissue detected at different time intervals following a single spray of cypermethrin formulation. Total cypermethrin indicates sum of four isomers labeled according to their elution time. Each value is an average of three replicate samples. Where no data is shown, indicates residues below the detectability limit.
Conclusion
Pyrethroid insecticides display a broad spectrum of insecticidal activity with low mammalian toxicity at low rates of application [16,17]. Two pyrethroid insecticides (permethrin and cypermethrin) were sprayed on beans and cucumber plants grown under greenhouse conditions to determine their dissipation rates and half-lives (T1/2 values). A gas chromatographic (GC) unit equipped with an electron capture detector (ECD) and a mass selective detector (MSD) were developed for identification and quantification of permethrin and cypermethrin residues simultaneously on beans and cucumber plants. The GC sensitive and reliable methods were capable of measuring the high disappearance rate of the two insecticides on cucumber fruits and leaves that have a high growth rate and multiple harvests. Permethrin degraded on cucumber leaves and fruits slowly compared to cypermethrin. Permethrin and cypermethrin undergo ester cleavage to produce the acid and alcohol moieties. Based on the MRL of 0.05µg g-1 fresh weight [14]. The reentry interval of permethrin required about 13 days to allow this insecticide to dissipate below its allowable limits, whereas the reentry interval of cypermethrin was only 6 days under greenhouse conditions. These results indicated that the period that elapse before harvesting (re-entry intervals) cucumber should be extended for permethrin due to the high growth rate and early maturity of cucumber fruits. Accordingly, to protect greenhouse workers from exposure to toxic pesticide residues, cypermethrin (not permethrin) could be selected for spraying cucumber due to its high dissipation rate and short half-life (T1/2) values. The T1/2 values of permethrin were 3.3 and 13 days on cucumber leaves and fruits, respectively. Whereas the T1/2 values of cypermethrin were 1.3 and 3.3 days on cucumber leaves and fruits, respectively indicating that permethrin decline comparatively slowly on cucumber fruits and leaves.
Acknowledgments
The author would like to thank Steven Diver and the University of Kentucky farm crew for their assistance in growing and spraying beans and cucumber plants under greenhouse conditions. The present investigation was supported by the United States Department of Agriculture, National Institute of Food and Agriculture (USDA/NIFA) Award # KYX-10-18-65P Accession No. 1017900 to Kentucky State University.
References
- Gallo D, Nakano O, Silveira Neto SS, Carvalho RPL, Baptista GC, et al., (2002) Entomologia agrícola. Piracicaba. Fundação de Estudos Agrários Luiz de Queiroz pp. 920.
- Rudolph R, Pfeufer E, Bessin R, Shawn Wright S, Strang J (2020) Vegetables Guide for Commercial Growers. University of Kentucky. College of Agriculture, Food and Environment, Cooperative Extension Service, ID-36.
- Berrada H, Fernandez M, Ruiz MJ, Molto JC, Manes J, et al., (2010) Surveillance of pesticide residues in fruits from Valencia during twenty months (2004/05). Food Control 21(1): 36-44.
- Afshar M, Salkhordeh N, Rajabi M (2013) An ecofriendly and stability indicating HPLC method for determination of permethrin isomers: application to pharmaceutical analysis. Journal of Chemistry 2013.
- Khater HF (2012) Ecosmart Biorational Insecticides. Chapter 2 In: Alternative insect Control Strategies. Insecticides Advances in Integrated Pest Management, Edited by: Farzana Per Veen. In Tech Janeza Trdine 9, 51000 Rijeka, Cortina, p. 17-60.
- Shafer TJ, Meyer DA, Crofton KM (2005) Developmental neurotoxicity of pyrethroid insecticides: Critical review and future research needs. Environmental Health Perspectives 113(2): 123-136.
- Page SW (2008) Antiparasitic Drugs. Chapter 10, page 241 In: Small Animal Clinical Pharmacology. Edited by Maddison, JE, Page SW, and Church, DB. Elvester Limited, second edition, Printed in China.
- Manna S, Bhattacharyya D, Basak DK, Mandal TK (2004) Single oral dose toxicity study of a-cypermethrin in rats. Indian J Pharmacology 36(1): 25-28.
- World Health Organization (Geneva) (1992) Alfa-cypermethrin. In: Environmental Health Criteria-142, WHO. Geneva: Library cataloguing in publication data; p. 20-43.
- US Department of Health and Human Services. Toxicological profile for pyrethrins and pyrethroids. U.S. Department of Health and Human Services, Public Health Service. Agency for Toxic Substances and Disease Registry, Division of Toxicology/Toxicology Information Branch, 1600 Clifton Road NE, Mailstop E-29, Atlanta, Georgia 30333 September 2003.
- O’Reilly AO, Bhupinder PS, Khambay MS, Williamson LM, Field BA, et al., (2006) Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem J 396: 255-263.
- Antonious GF, Hill R, Ross K, Coolong T (2012) Dissipation, half-lives, and mass spectrometric identification of endosulfan isomers and the sulfate metabolite on three field-grown vegetables. J Environmental Sci. Health, Part-B Pesticides 47(5): 369-378.
- Antonious GF, Turley ET, Mutari A, Snyder JC (2017) Dissipation, half-lives, and mass spectrometric identification of chlorpyrifos and its two metabolites on field-grown collard and kale. J Environ Sci. Health B 52(4): 251-255.
- FAO/WHO Joint Food Standards Program (1993) Codex Alimentarius Commission, volume 2, Pesticides Residues in Food, 2nd edition, section 2. Food and Agriculture Organizaton of the United Nations, World Health Organization, Rome, 1993.
- FAO/WHO Joint Meeting on Pesticide Residues, Pesticide residues in food (2018) Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. Berlin, Germany, p. 18-27.
- Ripley BD, Ritcey GM, Harris CR, Denomme MA, Brown PD (2001) Pyrethroid insecticide residues on vegetable crops. Pest Management Science 57(8): 683-687.
- National Institute for Standards and Technology (NIST). Mass Spectrometry Data Center Collection (electron ionization) by U.S. Secretary of Commerce, 2007.