Evaluation of the 3rd Generation of Backcrosses and its Parents of Two Bread Wheat (Triticum aestivum L.) Cultivars for Salt Tolerance
Dheya P Yousif*, Adel S. Hadi and Samer M Ahmed
Ministry of Science and Technology, Agricultural Research Directorate, Iraq
*Corresponding author: Dheya P Yousif, Ministry of Science and Technology, Agricultural Research Directorate, PO Box 765; Baghdad, Iraq
Article History
Received: November 12, 2020 Accepted: December 04, 2020 Published: December 07, 2020
Citation: Yousif DP, Hadi AS, Ahmed SM. Evaluation of the 3rd Generation of Backcrosses and its Parents of Two Bread Wheat (Triticum aestivum L.) Cultivars for Salt Tolerance. Int J. Agri Res Env Sci. 2020;1(1):04‒06. DOI: 10.51626/ijares.2020.01.00002
Abstract
A field experiment was conducted during the winter season of 2017-2018 at the Center of Plant Breeding and Genetics, Al-Tuwaitha Research Station (30km southeast of Baghdad) to evaluate the performance of two bread wheat genotypes at the 3rd back cross generation with their parents, cv. Furait , Baraka and Iraq under saline field condition (12 dSm-1 ). The objective of this study was to evaluate the beneficial effects of different back crosses and its parents in targeted field condition, on grain yield and its components of bread wheat. Results showed that the two generations of (Furait x Baraka) and (Furait x Iraq) were significantly exceeded their parents and gave the highest values of spikes m-2 (207.0 , 196.3), grain spike-1 ( 37.0, 39.0), 1000 seed weight (34.3, 33.3g ) and grain yield m-2 (244.4, 242.7g ), respectively. Phenotypic variation and the percentage of broad sense heritability for plant height, tillers m-2, grains spike-1, 1000 seed weight and grain yield m-2 were highest compared with the value of environmental variation, and emphasized the important of genotypic variation and the ability to improve the desirable quantitative traits and reflects the high percentage of heritability.
Keyword: Environment, Phenotypic variation, Heritability, Backcrosses and parents
Introduction
Plant growth and yield of bread wheat are seriously affected in salinity –prone environments, hence effective agricultural means are needed [1]. Bread wheat (Triticum aestivum L.) is a major food crop all over the world but increasable area are suffer from saline conditions annually. Therefore, increasing salinity tolerance for wheat is necessary due to its moderate salt tolerance with EC threshold of 6-8 dSm-1 (60-80 mM NaCl), [2]. According to Francois et al. [3], wheat yield is decreased by 3% for each increased unit of EC on field level. Salinity has affected the area cultivated with almost all crops all over the world [4]. High salt stress causes homeostasis change in water potential and ion distribution, molecular damage, growth inhibition and even death [5]. Salt stress adversely affects plant growth by osmotic stress, toxicity and nutrient deficiency [6]. Wheat breeders are interested to develop this strategic crop for salt tolerance and associated mechanisms in candidate cultivars [7,8].
Identification of salt tolerance mechanisms led plant breeders to develop new cultivars to reduce salinity problems [9]. While the progress has not been so impressive, screened many bread wheat cultivars for salt tolerance and summarized results of large international collections of wheat that have been screened by breeders in the hydroponic culture for wheat. Many Iranians were screened wheat accessions for grain yield at salinity condition in the field site in California [10] and no response for salt-tolerance. Hybridization is a useful tool for broadening the genetic variation within the crop species to estimate gene actions. Lyon’s [11] study on Lycopersicum is one of the first researches to evaluate the inheritance of salinity tolerance in a cross between Lycopersicon esculentum and L. pimpinellifolium which found that fruit yield in the hybrid was more affected by salinity than its parents. The objective of this study was to evaluate the beneficial effects of bread wheat back crosses at salinity stress conditions with its parents on growth parameters, grain yield and its components were studied.
Materials and Methods
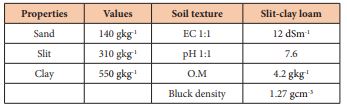
A Field experiment was carried out during the winter season of 2017-2018 at Al-Tuwaitha Research Station (30km southeast of Baghdad), Ministry of Science and Technology. Experimental field was prepared by plowing, disking and properly leveling and divided into plots of (2.0×1.5m). Two back crosses at BC3 generation for 2 wheat cultivars and its parents Furait (moderate salt tolerant), Baraka and Iraq sensitive local cultivars) for salt tolerance were planted on Dec. 15, 2017 in the agricultural field (12 dSm-1).The soil texture and it characterization showed in (Table 1). Nitrogen fertilizer was applied as recommended of N (200Kg ha-1) during planting and tillering (45 days after planting). Phosphorus fertilizer with 70kgha-1 of P2O5 superphosphate (16% P2O5) was added at planting [12]. All backcrosses with its parents were introduced in a yield trial with Randomized Complete Block Design with three replications. Grain yield, its components and some growth traits were measured. The phenotypic (σ2P), genotypic (σ2G) and environmental (σ2e) variances were estimated according to the method indicated by Snedecor and Cochran (1989). Data were subjected to analysis of variance and means were compared using LSD at P ≤0.05 by Gen stat statistical software program [13]. Broad sense heritability (H2B.S) was estimated ording to Nyquist et al., [14] which indicated that the heritability less than 40% considered as low, 40-60% was medium, and more than 60% as high.
Table 1: Physical and chemical properties of 0-40 cm of soil profile of Al-Twaitha Research Station during winter season of 2017/2018.
Results and Discussion
Analysis of variance
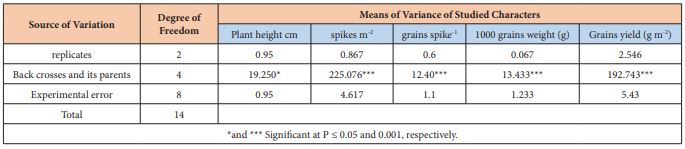
Table (2) showed that there were significant differences (P ≤ 0.05) among the backcrosses and their parent cultivars under investigation due to its wide genetic variation. On the other hand, the backcrosses produced generations are superior to their parents due to the heterotic pattern caused by genetically unrelated parents. Results agreed with Marzooghian et al [15] who emphasize on the high level of genetic variation and the possibility of conducting genetic analysis of the properties and estimation of the components of phenotypic variation.
Table 2: Mean squares of the analysis variance for bread wheat back crosses and their parents.
Effect of Salinity
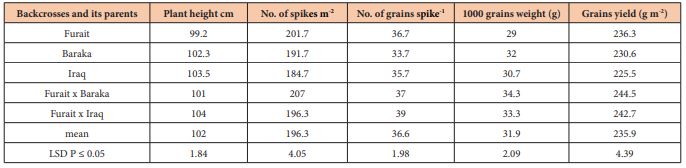
Plant height: Plant height was significantly affected (P ≤ 0.05) by different salinity stresses (Table, 3). All entries grown in salinity were shorter than the natural condition. Results obtained were agreed with Niaz et al. [16]. The two backcrosses exceeded its parents and gave plant height of 101.0 and 104.0 cm, respectively. Result agreed with Suiyun et al., [17].
Table 3: Means of backcrosses and its parents for grain yield and its component for bread wheat grown on salinity affected field (12 dSm-1) during 2017/2018 of Al-Twaitha .Res. Center, Baghdad, Iraq.
Spikes m-2: Table (3) reveals that there were significant differences among the backcrosses and its parents in the number of spike m-2 under the salinity stresses. Significant superiority for the two backcrosses than their parents which reflects the heterotic patterns of backcrosses on their parents. Backcrosses produced 207.0 and 196.3 spike m-2, respectively. Results agreed with Maas and Grieve [18] and Mass et al., [19].
Grain spike-1: The number of the grain spike-1 is an important quantitative trait as an essential grain yield component under salinity and/or normal environments. Results indicated that there were significant differences among the backcrosses and its parents in the number of grain spikes-1 and revealed the exceeding of back crosses on their parents and out yielded 37.0, 39.0 grain spike -1, respectively (Table 3). Results agreed with Houshmand et al. [20] concerned the most importance of this trait on the grain yield.
1000 seed weight: Table (3) showed that the two backcrosses affected in 1000 grain weight and gave 34.3g for (Furiat x baraka) and 33.3 g for (Furiat x Iraq). Grain weight is well documented to be a major yield component determining final grain yield in Mediterranean environments [21]. Results emphasize what [22] found in his study.
Grain Yield m2: Table (3) revealed that there were significant effects among the backcrosses and its parents on grain yield. The two backcrosses (Furiat x baraka) and (Furiat x Iraq) significantly exceeded on its parents and gave 244.5, 242.7 gm-2 in comparison with Furait, Baraka and Iraq, respectively (236.3, 230.6 and 225.5 gm-2. Results agreed with Turki et al. [23].
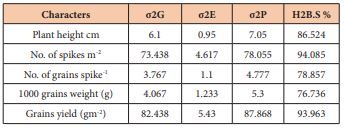
Table (4) reveald the estimation of the bread sense for all traits under investigation, Genotypic and phenotypic variability for grain yield were high in comparison with the low environmental variability (82.44, 87.87 and 5.43), respectively. The high values of broad sense heritability reflects the extent of the genetic base of plant genetic resources which plays an important role in deciding the suitability and strategy for selection [24].
Table 4: Genetic, environment and phenotypic variations for characters which had studied.
Broad sense heritability for spike m-2, grain spike-1 and 1000 grain weight were 94.08, 78.86 and 78.37, respectively which are considered as high as a genetic patameters for trait selection in breeding programs (Table 4). The highest heritability values indicate that heritability may be due to higher contribution of genotypic component and thus suggested that selection could be practiced with high genetic advance [25]. This results showed clear indication of the importance of genetic improvements in raising the efficiency of backcrosses and consistent with [26]. Results suggest that there is a high potential for inheriting the salinity characterization using the back-cross method as a coventional breeding method to overcome the increasing problem of salinity in Iraq [27-31].
Conclusion
Although salinity stress has been well documented as an effective parameter in decreasing crop growth rate and yield potential, developing and releasing new cultivars which are adaptable for salt tolerance can be a constructive program to overcome unsuitable environmental conditions. The present study indicated that it is possible to improve the salt tolerance in bread wheat by conventional backcrossing and transferring genes which are responsible for salt tolerance from moderate tolerated genotypes or cultivars to other having high yields and good quality but sensitive to salinity. Results reflected the success in obtaining new genotypes with good grain yield and salinity tolerance in the targeted region.
References
- http://www.iraq-icarda.org
- Hillel D (2000) Salinity management for sustainable irrigation: integrating science, environment, and economics. World Bank Publicat 315-327.
- Francois L, Maas E, Donovan T, Youngs V (1986) Effect of salinity on grain yield and quality, vegetative growth, and germination of semi-dwarf and durum wheat. Agronomy Journal 78: 1053-1058.
- Colmer T, Munns R, Flowers T (2006) Improving salt tolerance of wheat and barley: future prospects. Animal Production Science 45: 1425-1443.
- Zhu JK (2001) Plant salt tolerance. Trends in Plant Science 6: 66-71.
- Munns R, RA James, A Läuchli (2006) Approaches to increasing the salt tolerance of wheat and other cereals. Journal of experimental botany 57(5): 1025-1043.
- Sreenivasulu N, Grimm B, Wobus U, Weschke W (2000) Differential response of antioxidant compounds to salinity stress in salt‐tolerant and salt‐sensitive seedlings of foxtail millet (Setariaitalica). Physiologia Plantarum 109: 435-442.
- López-Aguilar R, Orduño-Cruz A, Lucero-Arce A, Murillo-Amador B, Troyo- Diéguez E (2003) Response to salinity of three grain legumes for potential cultivation in arid areas. Soil Science and Plant Nutrition 49: 329-336.
- Ashraf M, NA Akram (2009) Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Biotechnology advances 27(6): 744-752.
- Jafari-Shabestari J, Corke H, Qualset CO (1995) Field evaluation of tolerance to salinity stress in Iranian hexaploid wheat landrace accessions. Genetic Resources and Crop Evolution 42: 147-156.
- Lyon CB (1941) Responses of two species of tomatoes and the F1 generation to sodium sulphate in the nutrient medium. Botanical Gazette pp.107-122.
- Jadoaa KA, Hammed MS (2013) Fertilizer of wheat crop. National Program for Wheat Development. Nw-WD. Ministry of Agriculture. Paper 2, pp 12.
- Gen Stat Discovery Edition 4 (2013) Gen Stat Procedure Library Release PL18.2.
- Nyquist WE (1991) Estimation of heritability and prediction of selection response in plant– populations. Crist Rev Plant Sci 10: 235-322.
- Marzooghian A, Moghaddam M, Toorchi M, Shakiba MR (2014) Investigation of genetic structure and gene action in bread wheat affected by salt stress. International Journal of Biosciences (IJB) 5: 173-181.
- Niaz Ahmed Kalhoro, Inayatullah Rajpar, Shahmir Ali Kalhoro , Amjad Ali, Sajjad Raza, et al., (2016) Effect of salts stress on the growth and yield of Wheat (Triticum aestivum L.). American Journal of Plant Sciences 7: 2257-2271.
- Suiyun C, Suiyun G, Taiyong Q, Fengnin X, Yan J, et al., (2004) Introgression of salt-tolerance from somatic backcrosses between common wheat and Thinopyrim ponticum. Plant Sci 167: 773-779.
- Maas EV, Grieve CM (1990) Spike and leaf development of salt-stressed wheat. Crop Sci 30: 1309-1313.
- Mass EV, Lesch SM, Francos LE, Grieve CM (1994) Tiller development in salt- stressed wheat. Crop Sci 34: 1594-1603.
- Houshmand S, Zrzani A, Mirmohammadi Maibody SAM (2014) Effects of salinity and drought stress on grain quality of durum wheat, Commun. Soil Sci Plant Analysis 45:297-308.
- Timonova EM, Leonova IN, Röder MS, Salina EA (2013) Marker-assisted development and characterization of a set of Triticum aestivum lines carrying different introgressions from the T. timopheevii genome. Mol Breed 31: 123-136
- Adat-Noori SA (2005) Assessment for salinity tolerance through intergenetic hybridization Triticum durum×Aegilops speltoides, Euphytica 146: 149-155.
- Turki N, Harrabi M, Okuno K (2012) Effect of salinity on grain yield and quality of wheat and genetic relationships among durum and common wheat. J Arid Land Stud 22(1): 311-314.
- Kumar A, Mazzanti M, Mistrik M, Kosar M, Beznoussenko GV, et al., (2014) ATR mediates a checkpoint at the nuclear envelope in response to mechanical stress. Cell 158(3): 633-646.
- Larik AS, Hafiz HMI, Khushk AM (1989) Estimation of genetic parameters in wheat populations derived from intercultural hybridization. Pakphyton 1: 51-56.
- Hoffman AA, PA Parsons (1991) Evolutionary Genetics and Environmental Stress. Oxford Unit Press, New York pp.49-57.
- Genc Y, K Oldach, J Taylor, GH Lyons (2016) Uncoupling of sodium and chloride to assist breeding for salinity tolerance in crops. New Physiologist 210(1): 145-156.
- Maid LQ, Zhou EF, Huo NX, Zhou RH, Wang GY, et al., (2007) Genetic analysis of salt tolerance in a recombinant inbred population of wheat (Triticum aestivum L.), Euphytica. 153: 109-117.
- Munns R, M Gilliham (2015) Salinity tolerance of crops–what is the cost? New Phytologist 208(3): 668-673.
- Munns R, M Tester (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651-681.
- Snedecor GW, GW Cochran (1989) Statistical Methods, Eighth Edition, lowa State University Press, USA.